TGA hides from questions about sudden infant deaths after vaccination
A string of sudden unexpected deaths in infants following the Infanrix Hexa® vaccine has forced the drug regulator to go to ground.
Sudden unexpected death in infancy (SUDI) and sudden infant death syndrome (SIDS) are names for the sudden and unexpected death of a baby when there is no apparent cause of death.
The Therapeutic Goods Administration (TGA) has gone to ground after being confronted with questions about a series of sudden deaths in infants who received the Infanrix-Hexa®vaccine.
The “hexavalent” vaccine protects against six diseases (diphtheria, tetanus, whooping cough, polio, hepatitis B and Hib) and is administered to infants at 2, 4 and 6 months of age.
Approved by the TGA in 2006, the vaccine lies at the heart of the National Immunisation Program, and has been administered to millions of babies across the country.
FOI request
A freedom of information (FOI) request for the number of deaths reported after use of the Infanrix-Hexa® vaccine has revealed some worrying data.
The Database of Adverse Event Notifications (DAEN) shows 17 reported deaths in infants.
A further 26 reported deaths exist in the TGA’s ‘internal’ database, the Adverse Event Management System (AEMS), according to a recent FOI report.
Overall, 43 sudden unexpected deaths have been reported in babies mostly under 12 months of age, which have occurred within a day or two of vaccination.
Now, after many weeks of enquiries, the TGA has gone into hiding and refuses to confirm whether it has made any attempt to investigate the deaths.
Warnings from Europe
Infanrix-Hexa® was first authorised by the European Medicines Agency (EMA) in 2000, and the public has never been alerted to any safety issues.
EMA says it monitors pharmacovigilance data in the form of Periodic Safety Update Reports (PSURs), which are submitted by the manufacturer, GlaxoSmithKline (GSK).
Essentially, PSURs describe the worldwide safety experience of the vaccine over a defined period, and are not usually available to the public for independent scrutiny.
However, a major lawsuit in Italy involving GSK, resulted in the Judge ordering the drug company to publicly release its PSURs for the Infanrix Hexa® vaccine.
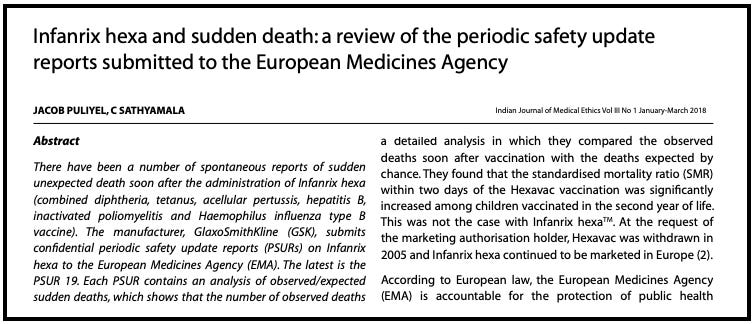
Those documents were sent to Jacob Puliyel, a paediatrician and Head of the Department of Paediatrics, St Stephen’s Hospital, Delhi, who carried out an independent review.
The analysis revealed a cluster of sudden deaths among infants less than 12 months of age -- 54 deaths (93%) occurred within the first 10 days of vaccination, and 4 deaths (7%) occurred within the next 10 days of vaccination.
Further, when he compared the rate of ‘expected’ sudden deaths, to the ‘actual’ rate of sudden deaths post-vaccination, there was a statistically significant increased risk of death in the first four days after vaccination, compared to the expected deaths.
The report concluded, “The clustering of deaths soon after immunisation suggests that the deaths were caused by the vaccine.”
Puliyel published the findings in the Indian Journal of Medical Ethics in 2018.
The report also showed that infant deaths, which were reported in the safety report (PSUR 16) were deleted in the PSUR 19, effectively underreporting the number of observed deaths in the final report seen by EMA.
I contacted Puliyel to ask why EMA had not raised the alarm regarding the PSUR data, and he said he thought the data were misleadingly presented to EMA.
“I wouldn’t go as far as saying that EMA colluded with GSK in the subterfuge, but I think EMA was negligent and accepted the manufacturers' deceptive data and interpretations unquestioningly,” he said.
Puliyel criticised EMA for its lax monitoring of post-marketing adverse events and has been urging all regulators to do better.
After the publication of his findings, Puliyel said there was no excuse for EMA to ignore the data discrepancies.
“The silence suggests EMA has no defence,” he remarked.
“I think nowadays, surveillance methods are designed to protect vaccine company profits rather than the public,” he added.
When I contacted EMA, the agency denied that deaths were “deleted” from the report as Puliyel claims.
Instead, EMA said the deaths were “reclassified” after it was determined the babies died of underlying diseases, such as “viral meningitis, an inborn error of metabolism congenital hydrocephalus and congenital heart disease.”
Puliyel rejected EMA’s explanation, calling it “singularly unconvincing.”
“Viral meningitis, congenital hydrocephalus and congenital heart disease would have been obvious at the time of vaccination when the children died – not discovered many years later,” explained Puliyel.
“EMA has to explain why these obvious underlying causes were not considered causes of death when the 16thPSUR report was published and why it had to be ‘reclassified’ years later,” remarked Puliyel.
'When the number of sudden deaths exceeded deaths expected as per the calculations in the 19th PSUR – there was this urge to ‘reclassify’ three sudden unexplained deaths as ‘deaths due to underlying causes,’” he said.
TGA enquiries continue
Efforts to compel a response from the TGA will continue, but the latest data on Infanrix-Hexa®have raised broader questions about the safety of the newer generation of vaccines designed to protect against multiple diseases within a single shot.
I will explore this in a forthcoming investigation.



No comments:
Post a Comment