New England Journal of Medicine: study confirms viral promoter with SV40 sequence causes cancer!
Skysona, a $3M/dose FDA-approved gene therapy causes cancer. Guess what it has in common with covid shots?
A recent paper published in the New England Journal of Medicine reported that BlueBird Bio’s gene therapy Skysona (aka eli-cel), approved by the FDA in 2022, causes cancer in approximately 10% of the treated population:
We performed integration-site analysis, genetic studies, flow cytometry, and morphologic studies in peripheral-blood and bone marrow samples from patients who received eli-cel therapy in two completed phase 2–3 studies…
…Hematologic cancer developed in 7 of 67 patients after the receipt of eli-cel…
Conclusions
Hematologic cancer developed in a subgroup of patients who were treated with eli-cel; the cases are associated with clonal vector insertions within oncogenes and clonal evolution with acquisition of somatic genetic defects.
In an editorial published alongside the data, Cynthia Dunbar, a hematologist at the NIH’s National Heart, Lung and Blood Institute, wrote that more cases may appear over time.
Even more interesting - it was known that eli-cel causes cancer before the drug was approved. In fact, it almost didn’t get approved and the company almost went out of business because of this.
In 2021 Bluebird Bio halted the trial for eli-cel after finding that the treatment likely caused a cancer-like condition in one patient. A boy with cerebral adrenoleukodystrophy (CALD) was diagnosed with myelodysplastic syndrome, a condition that can develop into leukemia, after receiving eli-cel. Two other patients were also being monitored for similar symptoms, and the FDA has put a clinical hold on eli-cel trials.
At the same time, the Cambridge based biotech also reported that it failed to reach an agreement with European governments. The German government offered ~$800K while the company wanted a $1.8M price tag.
Subsequently, Bluebird warned investors that its limited cash reserves, given its burn rate, raised “substantial doubt regarding its ability to continue as a going concern” and laid off 30% of its workforce.
Then, miraculously, the clinical trial hold due to cancer cases was lifted and Skysona was waved through by the FDA under an accelerated approval, meaning that Bluebird was allowed to sell its drug for $3M/treatment in the US, while the requirements for reporting full clinical trial results were pushed out to “after”. The approval was based on a post-hoc data analysis (a data fishing exercise) that managed to find a very minor improvement in functional scores at 24 months. This is as shaky as it gets. The FDA included a black box warning for the cancer “side effect”, but the company was free to sell the drug to desperate patients, while continuing to experiment on them.
CALD is marketed as a “rare neurodegenerative disease” with no known cause. According to Wiki, it hasn’t been pinned on a “genetic mutation”:
Clinically, ALD presents as a heterogeneous disorder, showing several distinct phenotypes, and no clear pattern of genotype–phenotype correlation.
While it’s been “linked” to a mutation of ABCD1 gene, the science is coy whether this mutation is present at birth or appears suddenly, out of the blue, after a “well baby visit” to the friendly pediatrician.
And:
It is characterized by normal development in early childhood, followed by rapid degeneration to a vegetative state…
Initial symptoms in boys affected with the childhood cerebral form of ALD include emotional instability, hyperactivity and disruptive behavior at school. Older patients affected with the cerebral form will present with similar symptoms. Untreated, cerebral ALD is characterized by progressive demyelination leading to a vegetative stateand death. …Onset of adrenal insufficiency is often the first symptom, appearing as early as two years of age
Oh! So, it’s a totally normal baby that sometime in early childhood develops a deadly brain/nervous system disorder, with absolutely no known cause, and degenerates to a vegetative state! I propose to study the incidence of CALD in correlation with “well baby visits” and vaccinations, and I can almost guarantee we can uncover the cause of it. But what do I know? I am not a smart scientist.
As I have written before, the trick of modern science is to proliferate names and classifications of “rare conditions” based on slight variations in presentation and diagnostic tests, as well as the age of the patients. Then pretend it’s a “rare mutation”, or an unknown cause, and proceed to finishing off these victims in the most expensive and profit optimizing manner.
According to Endpoint News, The authors of the NEJM study hypothesized that the cases of cancer may have been driven by the design of the lentiviral vector used in Skysona. Skysona’s claimed mechanism of action is “using a lentivirus to insert a functioning form of the ABCD1 gene”:
Skysona’s lentiviral vector was designed with the use of a viral promoter, intended to help drive strong expression of the gene. But a risk of that type of promoter is oncogenesis, or driving the development of cancer, Duncan [lead author] explained.
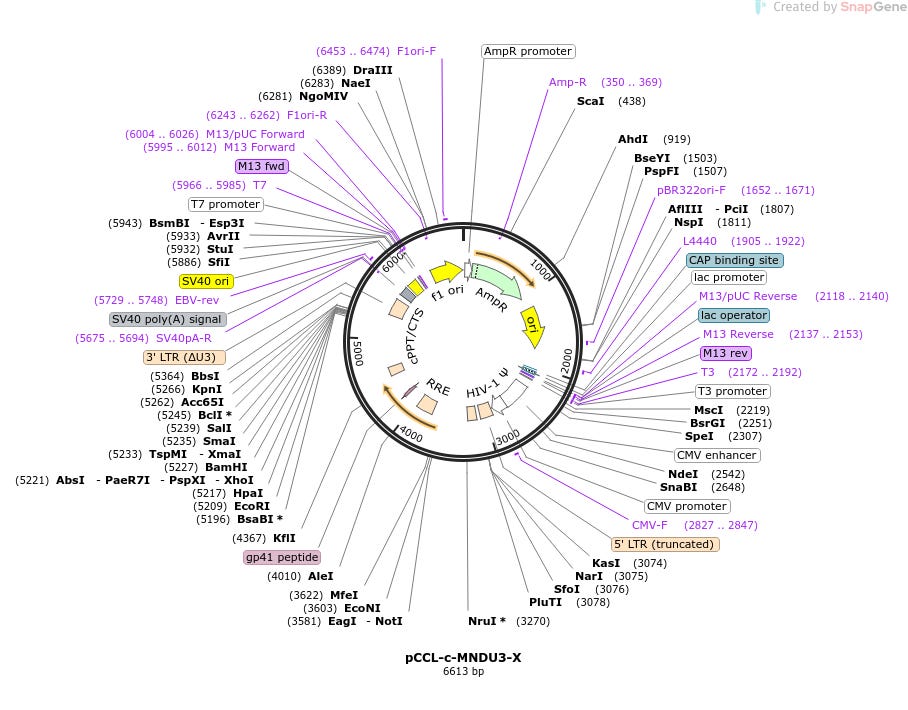
Mention of a “promoter” got me interested in tracking it down. The promoter used by Skysona is called MNDU3. I found it,
and … drumroll….
it has the famous SV40 sequence in it! (highlighted yellow on the left side):
Source: https://www.addgene.org/81071/
The NEJM article authors confirmed that the MNDU3 promoter which includes SV40 is indeed the causative agent of cancer:
“We’re relatively confident that that promoter in the design of the vector is driving this,” she said. “The question then is, in my mind, is it driving it alone, or are there other factors that contribute?”
Duncan noted that patients in one study, ALD-102, which has reported only one case of hematologic cancer, received a stronger chemotherapy regimen as part of preparation for treatment than another study, ALD-104, which reported the other six cases. But at this time, it remains unclear whether that made a difference, or whether other factors were involved.
Wait, what? A “conditioning regimen” of chemotherapy is required before this miracle of science can be administered? See p.3 of the drug label. It is required to nuke out the immune system and the body’s ability to produce red blood cells with chemo before the gene therapy will “work”. Maybe. A little. Or maybe it will give your child cancer. Well, as the biodefense expert Steve Hatfill says, “they are going to die anyway”!
This is another example of a new-new drug that requires treatment with OTHER DRUGS before it can be effective! I have written about this Potemkin village propping up the failed, toxic mRNA/gene therapy before:
The “therapy” is never studied separately from the “conditioning treatment”. Hence the NEJM authors have no idea why in a higher chemo dose group there were fewer cases of cancer post Skysona:
“Is it a combination of conditioning regimen and the vector?” she asked. “We can’t know for certain.”
Previous articles on gene therapies:
Art for today: The Flock, watercolor, 12x16 in.



No comments:
Post a Comment