The Bird Flu Vaccines - aren't coming; they’re here
Here's what's coming through that needle
Follow: Twitter | Instagram | Telegram | Truth Social | Podcast Membership|
Broadcasts: Rumble| HappyHour |This Wk wDrT | Brighteon | Bitchute | Tues Coffee
Learn about ECP: Health Center Cleveland, OH | Health Center Ventura, CA
Websites: DrTenpenny.com |Tenpenny Apparel| Tenpenny Supplements
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
I read a Twitter post last week that said the bird flu vaccines were already FDA-approved. So I did a little digging. Sure enough, as of the end of June 2024, the FDA licensed THREE bird flu (H5N1) vaccines:
Sanofi (for the National Stockpile).(package insert) Initially approved in 2007, it is an inactivated monovalent vaccine, indicated for persons 18 through 64 years of age. The recent trial included 151 enrollees: 103 adults were vaccinated, and 48 received a placebo. Four serious adverse events occurred after vaccination, including one death and three other SAEs (one each: menorrhagia (heavy menstrual bleeding), a cerebrovascular event, and breast cancer). It is made from eggs and inactivated by formaldehyde. The final product contains Triton X100, 500 mcg of porcine gelatin, 50 mcg of thimerosal (mercury), PEG, and sucrose. It contains no antibiotics and no latex. Similar to all other vaccines, this H5N1 vaccine has not been evaluated for carcinogenic or mutagenic potential, or for impairment of fertility.
ID Biomedical Corporation of Quebec (a subsidiary of GlaxoSmithKline).(package insert) This vaccine was actually approved in 2013 for use in children 6 months to 17 years. Two doses of 0.25 ml/shot for babies; persons 18 years and older are to get a 0.50 ml/shot. In the original vaccine trials, multiple severe adverse events were reported: a cerebral vascular accident on day 1 and day 9 following the second vaccine dose (1 subject), pulmonary embolism (1 subject) on day 21 following the first vaccine dose, and corneal transplant rejection (1 subject) 18 years post-transplant following the second vaccine dose. The vaccine, made from eggs, inactivated with formaldehyde, and disrupted with sodium deoxycholate (disrupts DNA) is supplied in two vials: one contains the H5N1 antigen in suspension; the other is a vial of AS03 adjuvant, the proprietary adjuvant containing squalene, polysorbate 80, and highly inflammatory DL-alpha- tocopherol. In fact, in 2009, during the H1N1 epidemic, an association was found between the AS03 adjuvant used in the shots and narcolepsy, a chronic neurological disorder that affects the brain's ability to regulate sleep-wake cycles.
The trial done with 838 children, 6 months to 17 years, had four times the rate of new immune mediated diseases in children given the vaccine containing AS03.
Seqirus. (package insert) Manufactured in Holly Springs, NC, is the third H5N1 vaccine that has already been approved under the trade name Audenz. Initially approved in 2020, the 0.5ml dose from a multi-dose vial is approved for all persons 6 months of age and older. The vaccine is made from MDCK cells (dog kidney), inactivated with B-propriolactone, and disrupted by the detergent cetyltrimethylammonium bromide (CTAB). It contains MF59, a squalene-based oil-in-water adjuvant that also contains the chemicals polysorbate 80, sorbitan trioleate, sodium citrate dihydrate, and citric acid monohydrate. Each dose 0.5ml dose also contains 25 mcg of mercury.
More On The Way
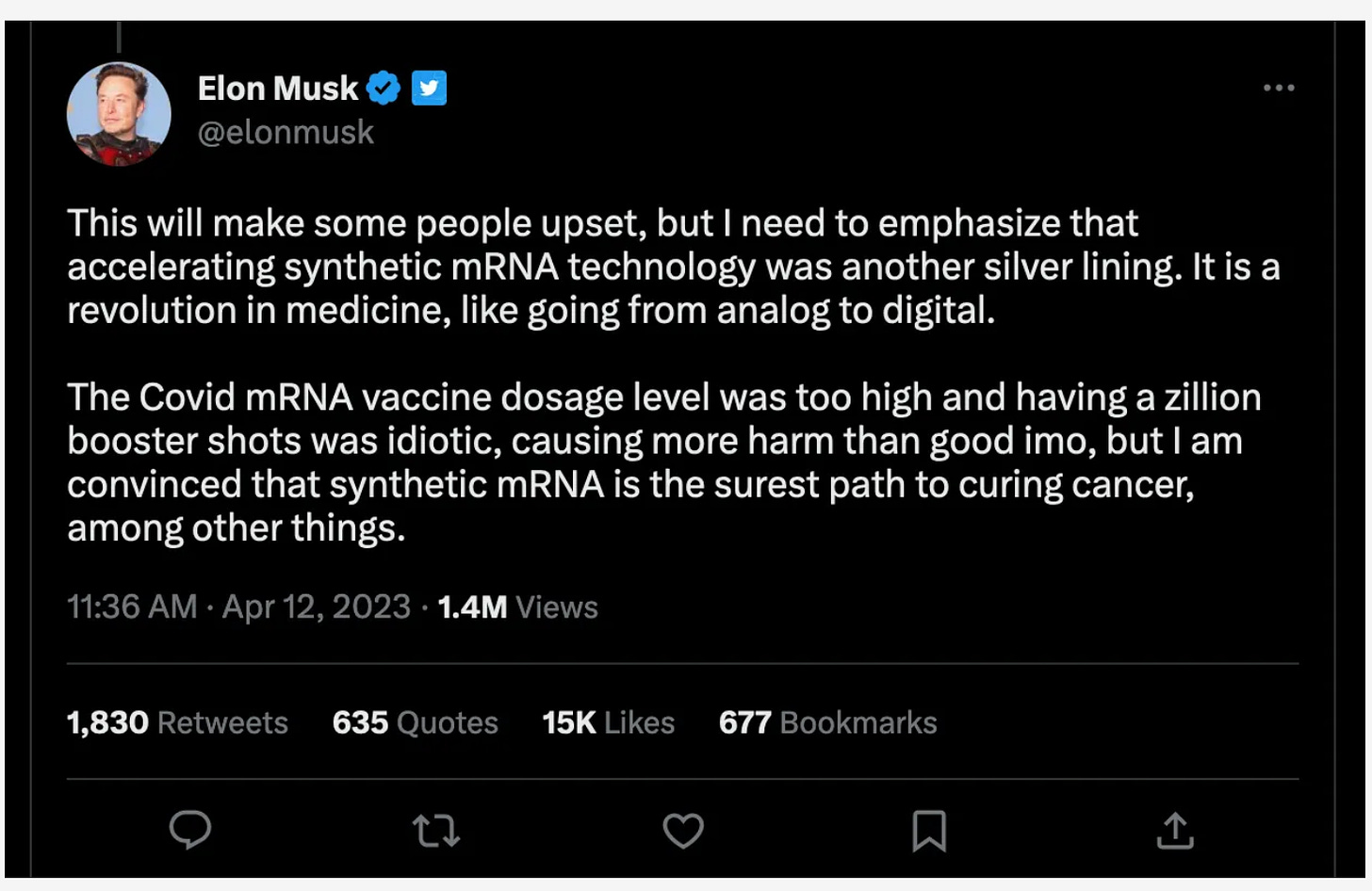
Moderna: The US government, through evil BARDA, paid the vaccine maker Moderna $176 million to accelerate the development of a pandemic influenza vaccine for bird flu. Moderna already has a bird flu vaccine in very early-stage testing that uses the same mRNA technology that allowed rapid development and rollout of vaccines to protect against COVID-19. But the project can be quicky redirected to target another form of influenza if a different threat than the H5N1 form of bird flu emerges. WATCH FOR H7N9 NEXT - it’s more dangerous.
They’re not wasting any time to plow ahead into the next plandemic.
And now this:





No comments:
Post a Comment