Finally, the fraud leading to COVID vax licensure and the supposedly legal imposition of vaccine mandates has been exposed by Congress
Rep. Thomas Massie's Subcommittee on the Administrative State released a 600 page report on the White House-FDA conspiracy to illegally license experimental (EUA) vaccine in August 2021.
I need to add: this report confirms that I was 100% correct that EUAs could not be mandated, that the White House and FDA believed this to be the case, and that the White House and FDA conspired to fraudulently regulate and license the vaccine, fraudulently mandate the unlicensed product, and fraudulently protect the government and manufacturer from liability. — Nass
https://judiciary.house.gov/media/press-releases/new-report-biden-administration-pressured-fda-and-ignored-risks-during-initial
NEW REPORT: Biden Administration Pressured FDA and Ignored Risks During Initial COVID Vaccine Phase
June 24, 2024
WASHINGTON, D.C. – Today, the House Judiciary Subcommittee on the Administrative State, Regulatory Reform, and Antitrust, led by Chairman Thomas Massie (R-KY), released an interim staff report titled, "Politics, Private Interests, and the Biden Administration's Deviation from Agency Regulations in the COVID-19 Pandemic.
"The report details how the Biden Administration pressured the Food and Drug Administration (FDA) to go beyond its regulatory authority to change its procedures, cut corners, and lower agency standards to approve the Pfizer COVID-19 vaccine and authorize boosters. This approval enabled the Biden Administration to mandate the COVID-19 vaccine, despite concerns that the same vaccine was causing injury among otherwise healthy young Americans.
"In August 2021, when the Pfizer shots received FDA licensure, and just before the booster received EUA, the top two FDA vaccine reviewers with decades of experience announced they were leaving the agency," said Chairman Thomas Massie (R-KY). "During the pandemic, politics overruled science at the government institutions entrusted with protecting public health. The FDA abandoned its congressional directive to protect citizens from false claims and undisclosed side effects, and instead ignored its own rules to pursue a policy of promoting the vaccine while downplaying potential harms. Exposing and acknowledging mistakes that were made is a necessary step toward restoring integrity and trust in our regulatory agencies."
The Subcommittee's investigation also revealed that the administrative state mishandled reports of vaccine injury,despite requirements to actively obtain, synthesize, and report feedback on the safety and efficacy of the Emergency Use Authorization (EUA) vaccine. Two former FDA scientists, Dr. Marion Gruber and Dr. Philip Krause, testified to the Subcommittee that they felt pressure to cut corners on the vaccine review, which was due to outside pressure to provide immediate approval so that the government could mandate vaccines. Despite evidence of harms from the EUA vaccine, the Biden Administration sought to fully approve the Pfizer vaccine through the Biologics Licensing Application (BLA) process.
Under the leadership of then-Acting FDA Commissioner Dr. Janet Woodcock, a long-time FDA staffer who the Biden Administration promoted to Acting Commissioner, and Dr. Peter Marks, head of the FDA's Center for Biologics Evaluation and Research (CBER), the agency cut corners in its usually rigorous BLA process to brand the Pfizer EUA vaccine as the only fully licensed "safe and effective" COVID-19 vaccine on the market at the time. Today, former Acting FDA Commissioner Woodcock says that, as it relates to vaccine-related injury, she is "disappointed in [her]self" and that the FDA did not do enough to address vaccine-related injury.
The FDA succumbed to the Biden Administration's pressure to act beyond its authority, which may have long-term impacts on the agency's ability to confidently serve the American public. This poor policy by the Biden Administration reveals many significant problems related to accountability and good decision making in the administrative state that warrant legislative reform. [Not to mention the million of lives harmed by the policy—Nass]
Read the full interim staff report and appendix here.
Hopefully this will help get some of the criminal conspirators into the defendant box.
I exposed this fraud the day the license was issued (August 23, 2021) and explained more about it with many additional posts over the next few days. Here are two of them:
Monday, August 23, 2021
I explain my theory of the COMIRNATY "license" scam by referring to passages in the FDA approval letter
Read the FDA approval letter please. This is how they scam you, by making it nearly impenetrable. Below, I identify the two most relevant passages. The rest of the letter is repetitive blather to keep your head spinning.
page 2 last line, footnote: here FDA quietly admits that the licensed Pfizer vaccine and the authorized Pfizer vaccine are identical w.r.t. safety and efficacy, but they are "legally distinct." That's code for one has manufacturer liability, while the other doesn't. It is also code for "we don't want to impose a mandate on the EUA product 'cause it is illegal, but we can probably get away with a mandate on the licensed product."
page 12 AA. This tells you that yes, we licensed the vaccine, but...there is a lot of the old vaccine out there, actually "a significant amount" and this amount will be considered an EUA vaccine and will continue to be used. Maybe for a very long time.
Now, why would they do that? Why specify that identical versions of the product will be legally different? Because they need the license to impose the mandates. But they need the EUA to evade liability.
Along with the license comes liability for the manufacturer. (But all EUA products were given a liability shield.)
Unfortunately, our federal overlords would prefer us to be without recourse if we are injured, rather than have Pfizer risk defending its product in court.
So, my inference is that the feds want the public to THINK the vaccine they are receiving is licensed, which will make people submit because they believe it can now be mandated, but instead the public is almost certain to receive the EUA vials instead, to save Pfizer's behind.
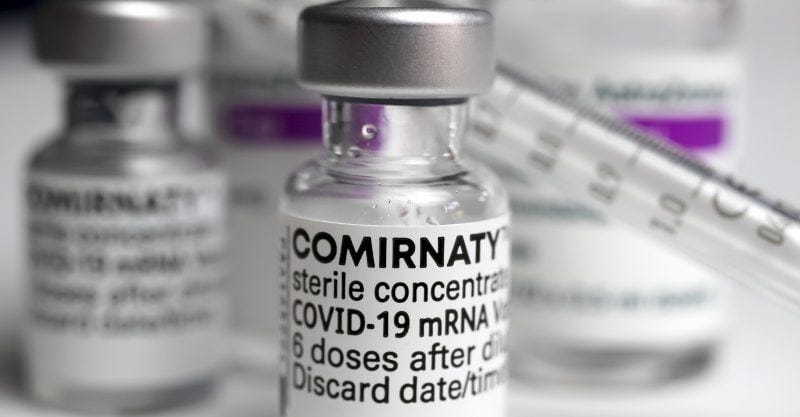
* You will be able to tell the difference when you see the vaccine vial. The letter explains that the COMIRNATY-labelled vials will be the licensed ones, and the others (under EUA) will say something like Pfizer BioNTech Covid-19 Vaccine withoutthe brand name COMIRNATY.
Yes, a stingy CICP injury program exists, which I have previously written about, but it has not paid out for a single Covid vaccine injury, last time I checked.
How did I figure this out? Because I have read other FDA approval letters, and this one had significant weasel wording. And I have seen other FDA tricks. So it just took me awhile to identify what was being hidden. I could be wrong. But then we would need another explanation for the language in the approval letter. I'm happy to entertain other interpretations.
P.S. Pfizer made $33 billion so far this year on its mRNA vaccine.
Posted by Meryl Nass, M.D. at 5:41 PM
and here is another, more polished:
Tuesday, August 24, 2021
The FDA's "approval" of Pfizer's Comirnaty vaccine is a Bait and Switch that makes mandates illegal
https://childrenshealthdefense.org/defender/mainstream-media-fda-approval-pfizer-vaccine/
2 Things Mainstream Media Didn’t Tell You About FDA’s Approval of Pfizer Vaccine
Buried in the fine print of Monday’s approval by the U.S. Food and Drug Administration of the Pfizer Comirnaty COVID vaccine are two critical facts that affect whether the vaccine can be mandated, and whether Pfizer can be held liable for injuries.
Monday, the U.S. Food and Drug Administration (FDA) approved a biologics license application for the Pfizer Comirnaty vaccine.
The press reported that vaccine mandates are now legal for military, healthcare workers, college students and employees in many industries. New York City Mayor Bill de Blasio has now requiredthe vaccine for all teachers and school staff. The Pentagon is proceeding with its mandate for all military service members.
But there are several bizarre aspects to the FDA approval that will prove confusing to those not familiar with the pervasiveness of the FDA’s regulatory capture, or the depths of the agency’s cynicism.
First, the FDA acknowledges that while Pfizer has insufficient stocks of the newly licensed Comirnaty vaccine available, there is “a significant amount” of the Pfizer-BioNTech COVID vaccine — produced under Emergency Use Authorization (EUA) — available for use.
The FDA decrees that the Pfizer-BioNTech vaccine under the EUA should remain unlicensed but can be used “interchangeably” (page 2, footnote 8) with the newly licensed Comirnaty product.
Second, the FDA pointed out that both the licensed Pfizer Comirnaty vaccine, and the existing vaccine are “legally distinct,” but proclaims that their differences do not “impact safety or effectiveness.”
There is a huge real-world difference between products under an EUA compared with those that FDA has fully licensed. EUA products are experimental under US law.
Both the Nuremberg Code and Federal Regulations provide that no one can force a human being to participate in this experiment. Under 21 U.S. Code Sec.360bbb-3(e)(1)(A)(ii)(III), “authorization for medical products for use in emergencies,” it is unlawful to deny someone a job or an education because they refuse to be an experimental subject. Instead, potential recipients have an absolute right to refuse EUA vaccines.
U.S. laws, however, permit employers and schools to require students and workers to take licensed vaccines.
EUA-authorized vaccines have an extraordinary liability shield under the 2005 Public Readiness and Preparedness Act. Vaccine manufacturers, distributors, providers and government planners are immune from liability. The only way an injured party can sue is if he or she can prove willful misconduct, and if the U.S. government has also brought an enforcement action against the party for willful misconduct. No such lawsuit has ever succeeded.
The government has created an extremely stingy compensation program, the Countermeasures Injury Compensation Program, to redress injuries from all EUA products.
The program’s parsimonious administrators have compensated under 4% of petitioners to date — and not a single COVID vaccine injury — despite the fact that physicians, families and injured vaccine recipients have reported more than 600,000 COVID vaccine injuries.
At least for the moment, the Pfizer Comirnaty vaccine has no liability shield. Vials of the branded product, which say “Comirnaty” on the label, are subject to the same product liability laws as other U.S. products.
When the Centers for Disease Control and Prevention’s (CDC) Advisory Committee for Immunization Practices places a vaccine on the mandatory schedule, a childhood vaccine benefits from an generous retinue of liability protections.
But licensed adult vaccines, including the new Comirnaty, do not enjoy any liability shield. Just as with Ford’s exploding Pinto, or Monsanto’s herbicide Roundup, people injured by the Comirnaty vaccine could potentially sue for damages.
And because adults injured by the vaccine will be able to show that the manufacturer knew of the problems with the product, jury awards could be astronomical.
Pfizer is therefore unlikely to allow any American to take a Comirnaty vaccine until it can somehow arrange immunity for this product.
Given this background, the FDA’s acknowledgement in its approval letter that there are insufficient stocks of the licensed Comirnaty, but an abundant supply of the EUA Pfizer BioNTech jab, exposes the “approval” as a cynical scheme to encourage businesses and schools to impose illegal jab mandates.
The FDA’s clear motivation is to enable Pfizer to quickly unload inventories of a vaccine that science and the Vaccine Adverse Events Reporting System have exposed as unreasonably dangerous, and that the Delta variant has rendered obsolete.
Americans, told that the Pfizer COVID vaccine is now licensed, will understandably assume that COVID vaccine mandates are lawful. But only EUA-authorized vaccines, for which no one has any real liability, will be available during the next few weeks when many school mandate deadlines occur.
The FDA appears to be purposefully tricking American citizens into giving up their right to refuse an experimental product.
While the media has trumpeted that the FDA has approved COVID vaccines, the FDA has not approved the Pfizer BioNTech vaccines, nor any COVID vaccines for the 12- to 15-year age group, nor any booster doses for anyone.
And FDA has not licensed any Moderna vaccine, nor any vaccine from Johnson & Johnson — so the vast majority of vaccines available in the US, if not all, remain unlicensed EUA products.
Here’s what you need to know when somebody orders you to get the vaccine: Ask to see the vial. If it says “Comirnaty,” it’s a licensed product. If it says “Pfizer-BioNTech,” it’s an experimental product, and under 21 U.S. Code 360bbb, you have the right to refuse.
If it comes from Moderna or Johnson & Johnson (marketed as Janssen), you have the right to refuse.
The FDA is playing bait and switch with the American public — but we don’t have to play along. If it doesn’t say Comirnaty, you have not been offered an approved vaccine.


No comments:
Post a Comment