Why we can't move forward with self-amplifying RNA technology
CQAs and potency data required
Everybody knows that the COVID-19 shots are associated with harms. We know this from pharmacovigilance and hospital data, peer-reviewed studies and on-the-ground reports of everything from cancer to autoimmune conditions. The exact mechanism of action of harm has yet to be definitively defined, but we do know that the harms are comprehensive and this points to immune system dysfunction.
A question on everybody’s minds is this:
How did it come to pass that billions of people were subjected to experimental gene-based prophylactic prodrugs wrapped in lipid nanoparticles when a plethora of deficiencies in critical quality attributes (CQAs) and potency remain even today?
Perhaps an even more relevant question is this:
How can it be that self-amplifying RNA-LNP technology which is based on exactly the same principles as the COVID-LNP product platform - plus some - is about to be pushed onto the Japanese public when none of these CQAs or potencies have been mitigated or even acknowledged by the entities pushing them?
Even if the manufacturers are trying to mitigate some of the issues, it is immoral and unethical and dare I say illegal, to do this post-facto. Think SV40. Think frameshifting. Think no dose.
Here are the principles of the RNA-LNP platform (and delivery) written as a goal:
The goal is to achieve a protective immune response with good immunological memory recall to prevent infection upon challenge with the ‘real-life’ pathogen using specific pathogen-related antigens. This response will depend on antigen expression. Antigen expression is proportional to the number of conventional mRNA transcripts successfully delivered [or produced] during “vaccination”. The number of RNAs successfully delivered depends on a multitude of factors including:
which and how many cells take up the LNPs?
the number of LNPs taken up by each cell?
the translation efficiency of the mRNA?
the % integrity of the mRNA?
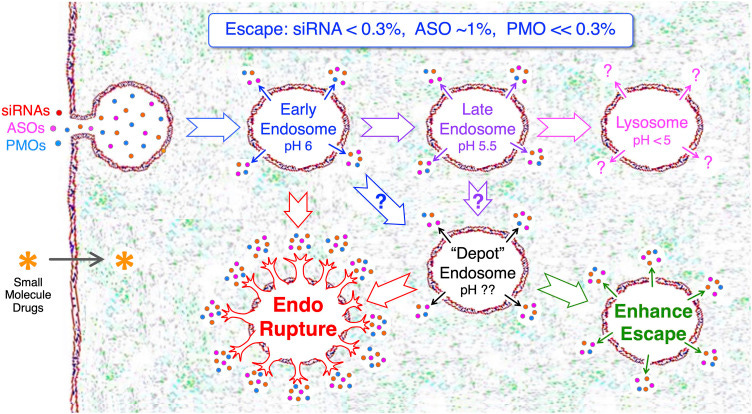
the rate of endosomal release of the mRNA?
just to name a few. The latter is perhaps the most important determining factor and is in fact the rate-limiting step. In biochemistry, the slowest step in a series of biochemical reactions or metabolic pathways is the rate limiting step.
Question:
How many of you know that according to in vitro studies, only 1-2% of all LNP-delivered nucleic acids (RNAs) make it to the cytoplasm of the cell? It begs the question, what effect does the 98% of the remaining foreign-introduced materials - with pathogenic potential - have on the cell? It remains inadequately studied.
Let me be blunt: There is no current way to determine how many mRNA transcripts will be successfully delivered to the cytoplasm and this is because the determining factors have not been characterized. Please take a moment to understand what I just wrote.
It means that there is no way to quantify a dose. It means that we don’t know from person-to-person how much, or which, protein will be made. It means that regardless of the so-called 30 ug “dose” of modRNA in a single Pfizer injection, we cannot know where the spike protein will be made, how much will be made, or for how long it will be made.
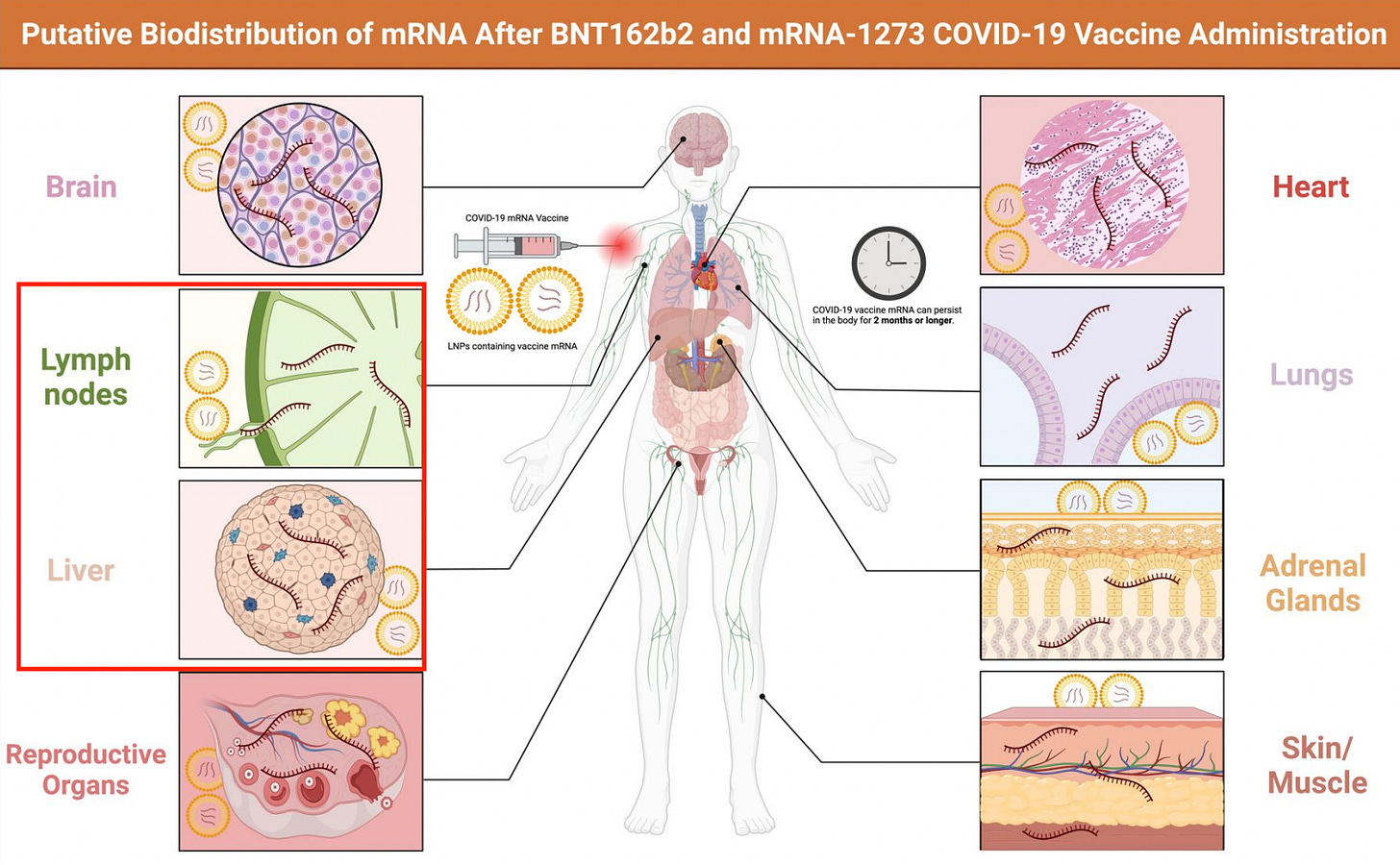
Besides the enumerable problems that are clearly linked to harms which include undefined mechanisms of action, problems associated with n1-methylpseudoU incorporation inducing frameshifting, process-related impurity issues like DNA contamination - it would be maniacal to move forward with a new product based on this platform, especially if this new product brings new problems. Which it does.
Enter self-amplifying mRNA
So consider that in the face of all of the problems we already know about (no thanks to the regulators, product pushers or governments), we now have to deal with the fact that the coding material itself contains a photocopier for the RNA. This means RNA copying will be autonomous within the cell yielding a potential endless amount of protein product, and don’t forget, this will be at the metabolic expense of the cell. The cell will die.
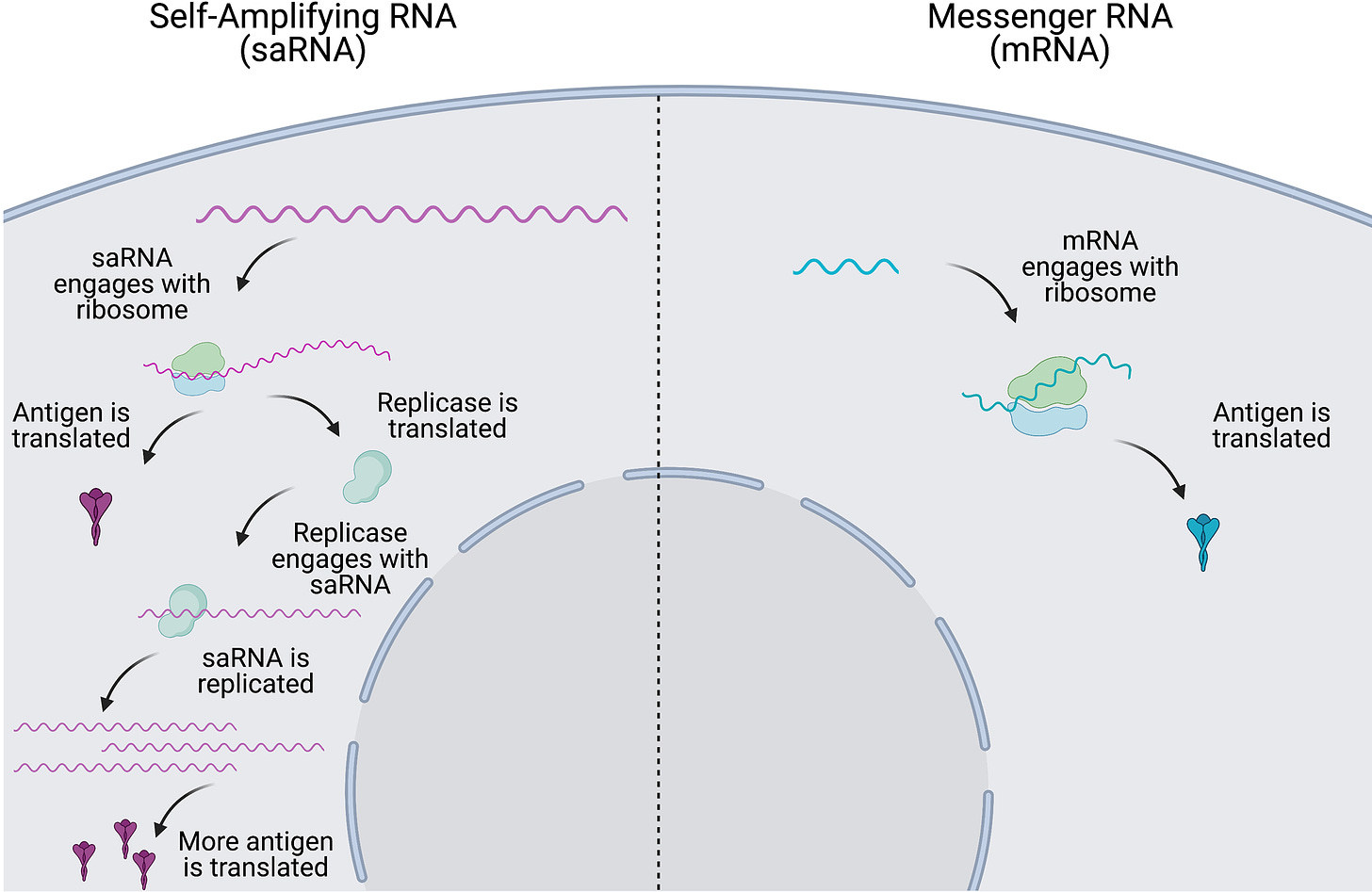
This will translate (pun intended) into more antigen being produced and for longer duration intracellularly and on the cell surface. All this excessive translation is very metabolically heavy, and all of that energy that should be going do the cell’s work will be going to making foreign proteins instead. For an unknown amount of time. The cell will die, or be killed by a cytotoxic T cell. Poor cell.
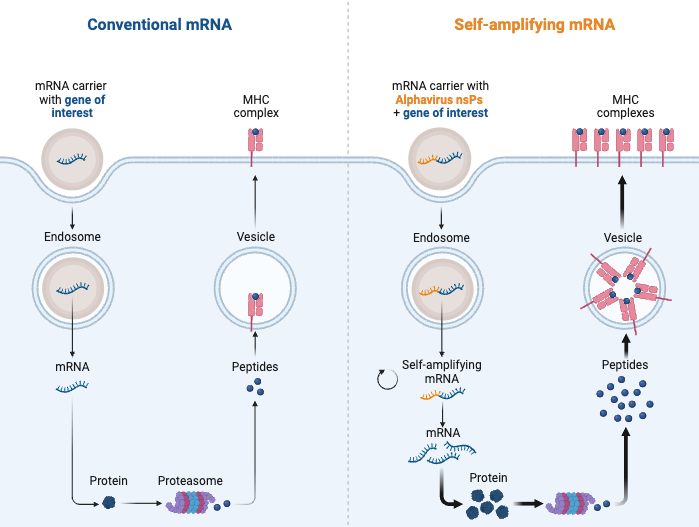
I think this is a very bad idea. It’s another example of absconding a beautiful mechanism used by Alphaviruses to propagate themselves, and abusing it in the biotech and experimental “vaccine” setting. This copy machine technology is the brainchild of certain Alphaviruses, like Venezuelan equine encephalitis virus (VEEV) for example, and although genius conceptually, I am exceedingly wary of tinkering with viruses and genetics for prophylactic prodrug “vaccine” design to counter viruses that aren’t harmful to people with in-tact immune systems.
Opinion: If this was meant to happen, we would have developed these abilities inherently.
Another exceedingly important point I would like to make is that the people who should be assessing environmental impact properly, aren’t, not for the “conventional” modRNA products, and not for these self-amplifying products, and as I currently understand it, this becomes really important in the context of the self-amplifying RNA LNP-based products because Alphaviruses can infect a whole bunch of different kinds of animals. Read this articlefor more information.
If it is also possible for recombination events between an endosome carrying RNA genetic material and an RNA virus to ensue, then what would be long-term effects on us and all the other animals that are susceptible to Alphaviruses?
If you want a point of entry for litigation purposes, take this to your MPs:
Until the QCAs and potency issues are resolved, no other products (including self-amplifying RNA products) based on genetic-LNP delivery systems should be released - not even at a local population level. Demand potency tests and IV (in process) expression tests and evidence of encapsulation efficiency by LNPs and sequencing of vials. Thanks for the chat Maria!
Use the known SV40 enhancer presence in the vials as proof of poor CQA assessment and use the dose issue - as in, there is no way to quantify a dose - to press the issue of safety and efficacy. Until a dose can be quantified (I honestly cannot think of how this could be done with current technologies), all injection regimes should be halted.
And save the vials you have. Do not destroy them. They are evidence.
The bottom line here is, if people partake in this new experiment under the guise of the “safe and effective” narrative umbrella every human and possibly many other creatures will be potentially and permanently affected. The question of exosome shedding from the “conventional modRNA” products has yet to be addressed, or assessed in any satisfactory way. Just how many endosomes are passed from the injected to the uninjected every day? I would really like to know.
THM: The tools and tests required as part of CQA and potency assessments are either not good enough, or not being utilized properly in order to draw conclusions pertaining to the long-term effects of both the modRNA or the self-amplifying RNA-LNP products; neither in the human context, nor in the environment and ecosystem context. To me, it’s beyond criminal that the powers that be intend to once again rush through to a potential disaster, especially since we are literally still in the midst of the first disaster, and potentially only reaping the tip of the iceberg of what was sown.
I think this new tech is an act against nature itself. Truly.
Mistakes were not made. Mistakes are not being made.
Dowdy SF. Endosomal escape of RNA therapeutics: How do we solve this rate-limiting problem? RNA. 2023 Apr;29(4):396-401. doi: 10.1261/rna.079507.122. Epub 2023 Jan 20. PMID: 36669888; PMCID: PMC10019367
Bitounis, D., Jacquinet, E., Rogers, M.A. et al.Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov 23, 281–300 (2024). https://doi.org/10.1038/s41573-023-00859-3
Munson, M.J., O’Driscoll, G., Silva, A.M. et al.A high-throughput Galectin-9 imaging assay for quantifying nanoparticle uptake, endosomal escape and functional RNA delivery. Commun Biol 4, 211 (2021). https://doi.org/10.1038/s42003-021-01728-8


No comments:
Post a Comment