Send This Article to People Who Say “Ivermectin Doesn’t Work for Covid-19”
If you hear your pharmacist, physician, or academic dean parrot the malignant regurgitated trope of “Ivermectin doesn’t workfor Covid” or that there is “no evidence” or “no data” to support ivermectin’s use in Covid-19, send them this meta-analysis summary and annotated bibliography of over 100 studies.
I never really latched on to the idea of social media, which is why I never signed up for it. In addition to pathological social factors, I think it is an especially absurd format for serious scientists to initiate a debate on the intricacies and complexities of medical research, clinical pharmacology, or patient care.
I did not have a Twitter/X account but very recently created one after I was contacted by colleagues alerting me to posts from Dr. Peter Hotez criticizing my recent testimony before the House Select Subcommittee on the Coronavirus Pandemic held by Dr. Brad Wenstrup (R-OH). Dr. Hotez is a pediatrician and tropical medicine Dean at Baylor in Houston, Texas. About six weeks later, Dr. Hotez responded to my testimony on Twitter/X:
I attempted to rebut Dr. Hotez’s statement by setting up a Twitter/X account only to find out that I couldn’t! Little did I know that the only way to comment on Dr. Hotez’s public Twitter/X page was to be granted permission by him to do so!! And here I thought the idea of Twitter was to foster discourse; not stifle it.
It certainly appears that dissenting scientific opinions are not welcome on Dr. Hotez’s Twitter/X page.
Dr. Hotez’s critique of my testimony was not an invitation to discuss the merits or shortcomings of ivermectin therapy; it was a figurative “drive-by shooting” stating that my sworn Congressional testimony was:
- “dangerous anti-science disinformation, pure and simple” and that
- “Ivermectin does nothing to help people with Covid” and
- “Ivermectin doesn’t work for Covid”
The second (in a list of around a dozen short, subsequent tweets by Dr. Hotez) was a pitch for his book.
Dr. Hotez then “upvoted” and reposted a note from one of his selected followers who appears to be a Twitter moderator of some sort. This individual had gleefully declared that my testimony had been “community-noted” adding that “Numerous valid scientific studies have shown that ivermectin is completely ineffective” (emphasis added) and “the promotion of Ivermectin for vaccine injury puts lives at risk.” The latter statement was a sleight of hand, as I had never opined on the use of ivermectin for “vaccine injury” at any time during my testimony or in any of my previous writings.
Twitter’s community notes are intended to give context to posts with debatable data, with this one, purporting to “debunk” my testimony, containing seven (7) reference links. Two of the links were duplicates, referring to the exact same data (numbers 1 and 3 and numbers 2 and 7). They referred to JAMA or NEJM studies which in turn have been criticized by academics as having very significant scientific and clinical practice shortcomings. Although the note additionally specified that “the promotion of ivermectin for ‘vaccine injury’ puts lives at risk,” none of those links determined that the use of ivermectin poses a safety “risk.” When prescribed correctly, ivermectin has not only been determined to be safe, but it has historically proven itself to be “astonishingly safe.”
The second-to-last “community note” link was a non-working link to an FDA website. It didn’t work because the FDA had agreed to delete it over a month earlier as part of a legal settlement for improperly denigrating ivermectin use. Didn’t the Twitter/X “community note” staffers bother to click on the links to make sure they worked before permitting them to be posted as references? Eventually, other individuals noticed the palpable shortcomings of the “community note” as well because it was quickly removed despite stating that it “Cites high-quality sources.”
A picture of the original “community note” with added arrows highlighting specific areas is shown below:
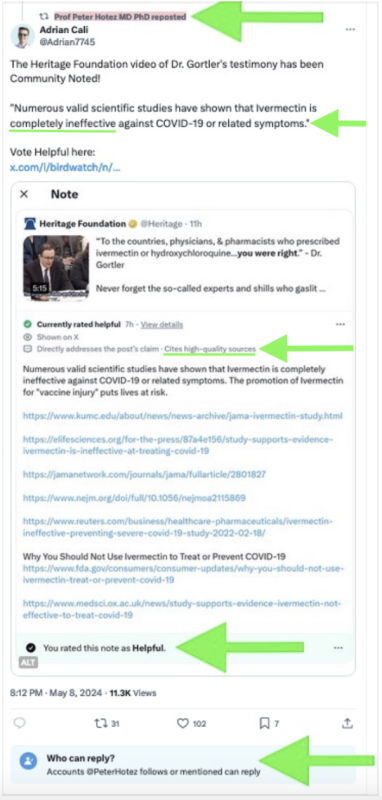
Of course, neither myself nor anyone else could contradict those claims because we were all blocked from posting by Dr. Hotez. It appears that he would rather make an incorrect assertion, then stick his fingers into his ears after he was finished saying what he had to, running away from any potential discussion, while his “approved” posters swarm to up-vote him – but all while potential counterarguments can’t be posted.
With no outside dissent allowed, does that mean Dr. Hotez “won” the debate?
It turns out my foray into Twitter was misguided and a waste of time. My Twitter/X account is now history. While it works great for a myriad of different matters, it is obviously an absurd place for a serious person to attempt to discuss or debate the intricacies of medical science or patient care. At this point, I have no intention of returning.
Ignoring and Censoring Data in History: Copernicus and Galileo
Consensus is very important to some, but unfortunately, it isn’t related to science. Science doesn’t care about consensus. In fact, many of the biggest scientific advancements were the result of questioning an established consensus. Generating a consensus for a new, controversial topic can be particularly dangerous. When people agree they tend to support each other, but a danger exists that they forget that they are reaffirming a potentially incorrect or polarized belief because their decision-making is biased and/or occurring in a vacuum.
In almost exclusively permitting harmonically positive feedback for himself, Dr. Hotez has failed to consider that it was artificially cultivated with essentially no meaningful dissent allowed to take place. In addition to being anti-free speech, it’s a terrible example for a scientist to set, particularly for someone in the position of a professor educating future scientists. The best scientists are the ones who are willing to listen to the opinions of other intellectuals and consider their arguments.
History shows us that ignoring scientific evidence and quashing dissent isn’t good for technical advancement; something that a professor who also labels himself a “science warrior” on his own homepage probably ought to already know.
A textbook example of “anti-science” was when Copernicus and Galileo tried to advance theories that the earth rotated around the sun (as opposed to the geocentric narrative of the earth being the center of the universe, around which all celestial objects rotated). Copernicus and Galileo were ignored and their writings were banned. Both were tried by a panel of their peers, found guilty, removed from their didactic pulpits, arrested and imprisoned. Galileo was eventually permitted to live out his remaining years, exiled under “house arrest” away on a farm. But even then, at least both Copernicus and Galileo were given opportunities to argue and present their evidence…unlike Dr. Hotez’s blockaded Twitter account.
Medicine is Rarely “Black or White”
Despite decades of advancement, clinical science is seldom black or white. Only very rarely are there declarations “never” or “nothing” or “completely.” Still, Dr. Hotez routinely makes polarizing, binary black-or-white, right-or-wrong assertions from his “members-only” perch on Twitter/X, neglecting or ignoring data – and it’s not just for Covid treatments.
A great deal of medical and pharmacology research deals with levels of uncertainty, something which I regularly taught my students and hoped that most medical scientists already knew and understood. Declarations otherwise would be irresponsible emerging from any medical scientist, let alone one with Dr. Hotez’s credentials.
Dr. Hotez’s unambiguous declarations that: ivermectin is “completely ineffective” and ivermectin’s use represents “anti-science disinformation pure and simple” are simply not reflected in both the review of many clinical trials and larger statistical analyses of published literature. In fact, there is data that sharply and directly contradict Dr. Hotez’s statement that “Ivermectin does nothing to help people with Covid.”
It is my belief that the continued accumulation of positive findings for ivermectin will continue and show an even greater positive effect for Covid pre-exposure prophylaxis, early exposure, and early treatment modalities. Like a good scientist, I am open-minded and am willing to hear out intellectual, alternative thoughts from my detractors. That being said, I have a considerable amount of data backing up my opinion.
Response to Dr. Hotez’s Assertion: “Ivermectin Does Nothing to Help People with Covid”
Since I am not permitted to respond on Twitter/X, (in addition to the fact that it is not an appropriate platform to discuss the complex details of clinical trial data) I’m responding in the form of a review, analysis, and lengthy, annotated bibliography.
Academics should encourage the discussion of controversial topics. In composing an argument, one needs to present all available data – not exclusively preferred findings from selected “big name” domestic medical journals (which by the way are often heavily financedwith expensive advertisements from Big Pharma) – but legitimate clinical and scientific data from all sources.
First, publications in “big name” journals like the NEJM and JAMA are not holy scripture beyond critique. Also, there is legitimate research being conducted in non-US countries and/or published in smaller journals worthy of consideration. On top of that, those who spend their lives in medical research will tell you that non-NEJM and non-JAMA, non-“big name” smaller, observational, and/or real-world study data are not only very worthy of consideration, but that those study designs and results can often be even more reflective of a drug’s utility and safety.
Cochrane’s Ivermectin Review is Incomplete
Cochrane’s March 2024 review of ivermectin has been cited as a source of data for ivermectin being ineffective. However, Cochrane only considered 11 Randomized Controlled Trials (RCTs) covering 3,409 participants. For ivermectin, there are 50 RCTscovering 17,243 participants which when analyzed in combination, show very strong evidence for efficacy in Covid-19. The fact that Cochrane selectively excluded a large amount of study data, while simultaneously including low-quality data with high conflict of interest and highly biased study designs, is more than a little perplexing. Of note, Cochrane also didn’t combine all of the evidence from the studies it did choose to include; data was split into very small sets by outcome and patient status, with no method used to combine all of the evidence from independent studies.
It seems to me that Cochrane isn’t what it used to be, and I for one am very disappointed.
Data Analysis
The proper application and weighting of data and its random effects meta-analysis across all studies can provide a complete picture of an effect. It includes all data, including too-low-dose, too-short-duration, less effective late- versus early-treatments, wrongly used fasting dosing (ivermectin is substantially better absorbed with high-fat foods [2.5x greater] according to the Merck package insert).
To date, there exists a list of 103 manuscripts written which studied ivermectin. Those data also include 15 medRxiv and/or preprint articles, (for journals that refuse to defy Big Pharma narratives and/or potentially those that the White House has ordered censoring) and all studies that showed ivermectin’s effectiveness with some certitude, but not at the level of p≤0.05. On a side note: inclusion versus exclusion of the medRxiv/preprint articles does not alter the overall positive ivermectin treatment effects.
These clinical findings are in addition to the highly plausible molecular biology and pharmacology mechanisms of how ivermectin is potentially effective for preventing the entry of some viruses into cells. For purposes of keeping the length of this document manageable, the pharmacologic mechanism of action will not be discussed here.
A Value of p ≤0.05 is Important, but it Isn’t Everything
Studies with p-values higher than 0.05 still provide evidence – just evidence with a lower than 95% confidence. Alone, those studies may not provide statistical confidence by themselves against the null hypothesis. However, they may contribute to a meta-analysis, in which they are weighted appropriately. In an analysis, they may actually result in strong statistical evidence and greater confidence from the combination of data from multiple independent scientific teams. Smaller studies and real-world observational studies should not always be dismissed as non-evidential; even case reports and case series have historically played an important role in biomedical research and the assessment of drug safety. In fact, those sources of data were part of what I routinely considered in approving new drugs and labeling updates during my years at the FDA as a drug safety expert.
RCTs are conceptually preferred if properly designed and carefully conducted, but the Covid era exposed severe biases in such trials: including but not limited to treatment delays (as Covid-19 along with any antiviral treatment must begin promptly) protocols that were designed to fail, mid-study changes, biased analysis and presentation, and lack of transparency in data and suspiciously timed publication releases. Each study should be evaluated for potential biases and/or confoundings on its own merit, whether randomized or observational, large or small.
Major RCTs allegedly producing Big Pharma-coined-term of “Evidence-Based Medicine™” published in “big journals” can appear very compelling, especially because they are what the lay press tends to cite most commonly, but clinicians should know that it is important to examine the methodology used beyond the high-level summary overviews and to also look at additional sources of data.
Another problem with RCTs is that, unlike real-world and observational studies, not just anyone can conduct large RCTs. Barriers include them often being significantly more expensive, time-consuming and requiring a dedicated, highly skilled support staff. Those sorts of requirements prohibit entry by less-well-funded clinicians who have smaller practices/facilities or those that have employment requirements which have a focus on direct care responsibilities as opposed to clinical research. While federal grants are available, they are highly competitive and tend to be limited to particularly listed topics which in turn end up being awarded to a limited number of major centers with those aforementioned resources.
Those major centers and/or their employees can be connected in one way or another to Big Pharma funding. For highly profitable Covid-19 drug trials, it could directly or indirectly create a conflict or incentive to show a lack of effectiveness or safety for inexpensive generic products, and in turn show efficacy for novel, expensive patented commercial products. This scenario not only applies to Covid-19 treatments such as ivermectin, it applies to a fair amount of all investigational medicine research.
In fact, a multitude of smaller, less expensive non-RCT observational/real-world-use studies across many facilities can make a stronger case by noting that dependence on any individual trial is subject to potential confounding, errors, bias, and even fraud. Therefore, the combined evidence from multiple, well-designed and conducted smaller, real-world, case reports, case series, and/or observational trials from an array of smaller facilities, combined via meta-analyses can sometimes be a stronger indicatorthan that of just one or a few biased large trials.
A diagram adapted from a Nature publication(below) illustrates a scenario in which 4 (four) smaller studies that individually may not have delivered statistical significance (ie, have a p>0.05) but when considered in combination, may provide strong evidence with a statistical significance via meta-analysis:
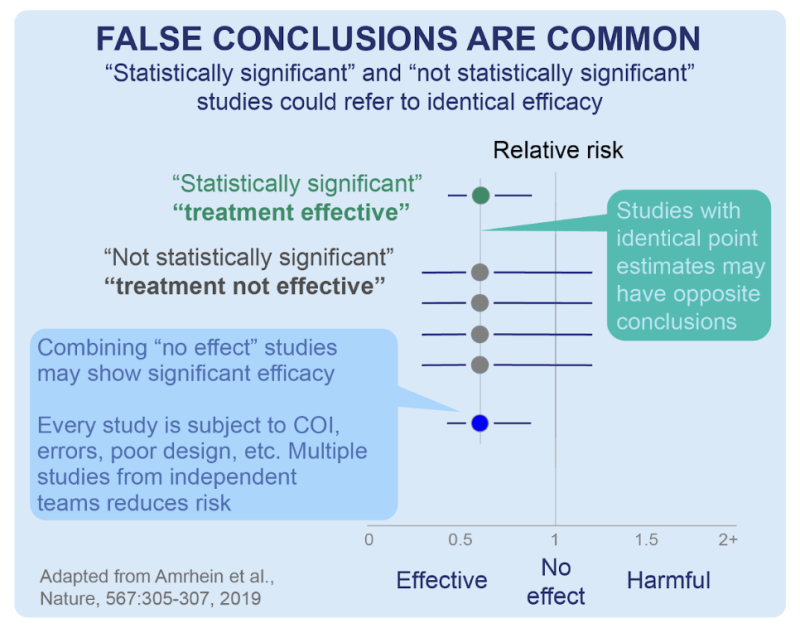
Separately, that same publication additionally underscores how important it is for scientists and clinicians to not mistakenly assume that “non-significance” (ie, a higher deviation from p≤0.05) translates to “no effect.” Statistical significance is just a numerical estimate of the confidence of a result. The idea that a small p-value implies that the estimate is credible/true/valid /the-only-thing-that-matters is a misconception. A small p-value of an RCT (for instance) says nothing about the quality of the estimate.
In the matter at hand, and in summation, a random-effects meta-analysis shows a clinically beneficial effect of ivermectin with a certainty of p<0.00000000001 (that is, one in one sextillion) over all 103 ivermectin studies for Covid-19, and also for RCTs and for specific outcomes like mortality hospitalization and recovery cases which all show p<0.0001.
Timing is Everything…(When it Comes to Initiating Antiviral Treatment)
The use of the word “early” in the “c19early.com” website is an important annotation. It reminds us of how critical timing is when it comes to any antiviral/antimicrobial drug administration. Ivermectin as an antiviral works best when administered early upon symptom(s) (or for prophylaxis/pre-exposure). That is the same when it comes to any antiviral pharmacology treatments, including for cold sores, genital herpes, influenza, or HIV/AIDS for instance.
Delayed administration could still have a clinical benefit, but less so, depending on how late and individual factors that include viral replication, infective loading dose, and viral variant/mutation, besides numerous demographic, immunologic, plus other factors. That is a fundamental concept that anyone in the field of pharmacy or medicine should have learned early in their schooling, yet it seems to have been omitted in about half of the 103 studies done on ivermectin which employed delayed or late treatment.
In addition to the delay in ivermectin dosing was the delay in releasing study findings. The worst example might be PRINCIPLE RCT results which were delayed over 800 days from the expected release of findings. PRINCIPLE (bibliography and explanation in reference number 88 below) was biased against showing efficacy per the design, operation, analysis, and reporting, and including very late ivermectin administration, yet still ended up showing a positive effect of ivermectin. During the delay in releasing data, novel, expensive, likely less efficacious “rebounding” Big Pharma treatments like molnupiravir and Paxlovid were developed, (and tested against placebo instead of treatments like ivermectin) reviewed, authorized, and White House-endorsed. Paxlovid ($1,400 per treatment course) and molnupiravir ($700 per course) were each around ten times more expensive than ivermectin (<$100 per course). Paxlovid purchased by the White House cost American taxpayers over $10 billion.
For perspective: the greater than $9 billion savings from the use of ivermectin alone could have instead bought about 36,000 $250,000 Lamborghini Huracans, or alternatively for those of us who must work for a living, about 300,000 $30,000 Toyota Camry SEs (the most popular model).
For Covid-19, There is More to the Data than Just Press/Abstract “Topline Results”
To fully address transparency, I am including a full list of ivermectin studies completed to date, with the plurality of positive and negative findings in the form of an annotated bibliography at the end of this article to allow readers to see the sources of the research. Each of the 103 references includes a brief summary and a link to a longer analysis at c19early.
Along with the bibliography, I am also including two summary plots of the ivermectin data from c19early on overall benefit, and relative benefits from prophylaxis, early, and late treatments.
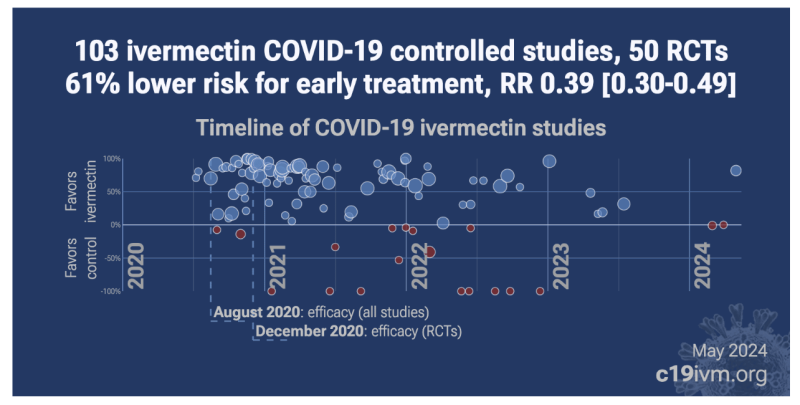
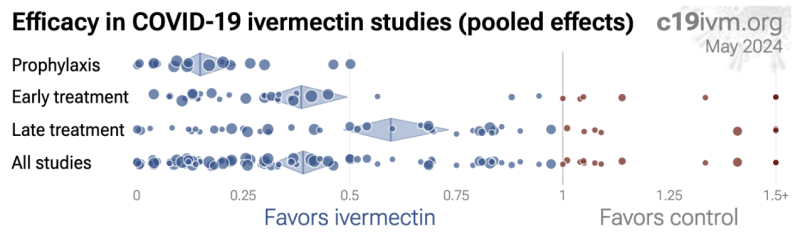
In the pictures shown above, the BLUE circles shown are studies that detail positive ivermectin study findings and the RED circles are negative. Negative data exists, but the positive ivermectin findings outnumber them both in study quantity and study size (illustrated by the circle sizes), as well as over time and by indication, according to meta-analysis data published at: c19ivm.org.
DISCLAIMER: Do NOT discontinue or initiate taking ANY drug without first discussing it with a pharmacist or physician you know and trust.
Annotated Bibliography
1. R. Chahla, L. Medina Ruiz, E. Ortega, M. Morales, F. Barreiro, A. George, C. Mancilla, S. D’ Amato, G. Barrenechea, D. Goroso, and M. Peral de Bruno, Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina Jan 2021, American J. Therapeutics, Volume 28, Issue 5, Page e601-e604
234 patient ivermectin prophylaxis RCT: 95% fewer moderate/severe cases (p=0.002) and 84% fewer cases (p=0.004).
Prophylaxis RCT for ivermectin and iota-carrageenan in Argentina, 117 healthcare workers treated with ivermectin and iota-carrageenan, and 117 controls, showing significantly lower cases with treatment. There were no moderate/severe cases with treatment vs. 10 in the control group. There were 4 cases with treatment (all mild) vs. 25 for the control group. https://c19p.org/ivercartuc
2. R. Mahmud, M. Rahman, A. Iftikher, K. Ahmed, A. Kabir, S. S. K. Jakaria Been, R. Mohammad Aftab, F. Monayem, I. Shahidul, I. Mohammad Monirul, B. Anindita Das, H. Mohammad Mahfuzul, M. Uzzal, Y. Mohammad Abdullah, M. Hossain, Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial Oct 2020, J. Int. Medical Research
EARLY TREATMENT 366 patient ivermectin early treatment RCT: 86% lower mortality (p=0.25), 57% lower progression (p=0.001), 94% improved recovery (p<0.0001), and 39% improved viral clearance (p=0.002).
RCT for ivermectin+doxycycline showing improvements in mortality, recovery, progression, and virological cure. 183 treatment and 183 control patients with no deaths in the treatment arm vs. 3 in the control arm (the 3 control deaths are not included in the analysis of other outcomes). Results may reflect the use of ivermectin, doxycycline, and potential synergistic effects of the combination. In the PRINCIPLE trial, no mortality benefit was seen for doxycycline alone (0.6% mortality with doxycycline vs. 0.2% control). https://c19p.org/mahmud
3. V. Desort-Henin, A. Kostova, E. Babiker, A. Caramel, R. Malamut, The SAIVE Trial, Post-Exposure use of ivermectin in Covid-19 prevention: Efficacy and Safety Results Jan 2023, ECCMID 2023
399 patient ivermectin pre-exposure prophylaxis RCT: 96% fewer cases (p<0.0001).
pre-exposure prophylaxis RCT 399 patients in Bulgaria showing significantly lower Covid-19 cases with ivermectin prophylaxis, and significantly lower cases with high viral load. No participant had severe symptoms, required oxygen, or was hospitalized. All patients with Covid-19 were treated with vitamin C and vitamin D. This trial makes the Cochrane analysis report statistically significant efficacy for prophylaxis, although Cochrane does not appear to have acknowledged this yet. There are currently 4 prophylaxis RCTs, and all 4 show statistically significant efficacy of ivermectin. Cochrane ignored them by simply choosing to only include post-exposure prophylaxis RCTs, even though they were included for the paxlovid analysis with many of the same authors. At the time there were no post-exposure RCTs and they knew that including any one of the 3 pre-exposure prophylaxis RCTs would show statistically significant efficacy. https://c19p.org/desorthenin
4. M. Varnaseri, F. Amini, R. Jamshididan, M. Dargahi, N. Gheibi, S. Abolghasemi, M. Dayer, N. Varnasseri, K. Hoseinynejad, S. Kheradhoosh, P. Nazari, E. Babadi, S. Mousavinezhad, and P. Ebrahimi, Ivermectin as a Potential Addition to the Limited Anti-COVID-19 Arsenal: A Double-Blinded Clinical Trial Apr 2024, Jundishapur J. Health Sciences, Volume 16, Issue 2
LATE TREATMENT 110 patient ivermectin late treatment RCT: 82% lower ventilation (p=0.02), 83% lower ICU admission (p=0.0004), 33% shorter hospitalization (p=0.001), and 28% faster recovery (p<0.0001).
Double-blind RCT 110 hospitalized moderate to severe Covid-19 patients showing significantly reduced ICU admission, shorter hospitalization, faster resolution of symptoms, and improved CRP and LDH levels with ivermectin treatment compared to placebo. No deaths occurred in either group. There were no serious adverse events. Note that preclinical research predicts synergistic effects with the standard treatment protocol used in both groups. https://c19p.org/varnaseri
5. A. Biber, G. Harmelin, D. Lev, L. Ram, A. Shaham, I. Nemet, L. Kliker, O. Erster, M. Mandelboim, and E. Schwartz, The effect of ivermectin on the viral load and culture viability in early treatment of non-hospitalized patients with mild COVID-19 – A double-blind, randomized placebo-controlled trial Feb 2021, Int. J. Infectious Diseases
EARLY TREATMENT 89 patient ivermectin early treatment RCT: 70% lower hospitalization (p=0.34) and 62% improved viral clearance (p=0.02).
Double-blind RCT for mild-moderate Covid-19 outpatients in Israel showing significantly faster reduction in viral load with treatment, and lower hospitalization with treatment. The one treatment hospitalization was a few hours after treatment and the patient improved and was discharged quickly. Authors also examine culture viability on days 2-6, with 13% positive in the ivermectin group vs. 48% in the control group. There were no safety issues. Ivermectin was taken one hour before a meal, reducing concentration. https://c19p.org/biber
6. R. Chahla, L. Ruiz, T. Mena, Y. Brepe, P. Terranova, E. Ortega, G. Barrenechea, D. Goroso, and M. Peral de Bruno, Randomized trials – Ivermectin repurposing for COVID-19 treatment of outpatients with mild disease in primary health care centers Mar 2021, Research, Society and Development, Volume 11, Issue 8, Page e35511830844
EARLY TREATMENT 254 patient ivermectin early treatment RCT: 87% higher hospital discharge (p=0.004).
Cluster RCT outpatients in Argentina showing significantly faster recovery with ivermectin. There were no deaths. Outpatients in Tucumán were assigned to the ivermectin group and outpatients from San Miguel de Tucumán and Gran San Miguel de Tucumán were assigned to the control group. All comorbidities, percentage of male patients, and age were higher in the ivermectin group, favoring the control group. https://c19p.org/chahla
7. M. Khan, M. Khan, C. Debnath, P. Nath, M. Mahtab, H. Nabeka, S. Matsuda, and S. Akbar, Ivermectin treatment may improve the prognosis of patients with COVID-19 Sep 2020, Archivos de Bronconeumología, Volume 56, Issue 12, Page 828-830
LATE TREATMENT 248 patient ivermectin late treatment study: 87% lower mortality (p=0.02), 89% lower ICU admission (p=0.007), 83% lower progression (p=0.0004), and 87% improved recovery (p=0.02).
Retrospective 115 ivermectin patients and 133 control patients showing significantly lower death and faster viral clearance. Some potential issues and the authors’ response can be found in [sciencedirect.com, sciencedirect.com]. https://c19p.org/khan
8. Z. Aref, S. Bazeed, M. Hassan, A. Hassan, A. Rashad, R. Hassan, and A. Abdelmaksoud, Clinical, Biochemical and Molecular Evaluations of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Reducing Upper Respiratory Symptoms of Mild COVID-19 Jun 2021, Int. J. Nanomedicine, Volume Volume 16, Page 4063-4072
EARLY TREATMENT 114 patient ivermectin early treatment RCT: 63% improved recovery (p=0.0001) and 79% improved viral clearance (p=0.004).
RCT 114 patients in Egypt, 57 treated with ivermectin mucoadhesive nanosuspension intranasal spray, showing faster recovery and viral clearance with treatment. NCT04716569. https://c19p.org/aref
9. A. Chowdhury, A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients Jul 2020, Eurasian J. Medicine and Oncology
EARLY TREATMENT 116 patient ivermectin early treatment RCT: 81% lower hospitalization (p=0.23), 46% improved recovery (p<0.0001), and 81% improved viral clearance (p=0.23).
Small 116 patient RCT with low-risk patients comparing ivermectin+doxycycline and hydroxychloroquine +AZ, showing lower hospitalization, higher viral clearance, and faster symptom resolution and viral clearance with ivermectin+doxycycline. Mid-recovery resolution of symptoms is statistically significantly better with treatment, while other measures do not reach statistical significance. Instructions were to take ivermectin on an empty stomach, reducing lung tissue concentration. https://c19p.org/chowdhury
10. S. Ahmed, M. Karim, A. Ross, M. Hossain, J. Clemens, M. Sumiya, C. Phru, M. Rahman, K. Zaman, J. Somani, R. Yasmin, M. Hasnat, A. Kabir, A. Aziz, and W. Khan, A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness Dec 2020, Int. J. Infectious Diseases, Volume 103, Page 214-216
EARLY TREATMENT 72 patients ivermectin early treatment RCT: 85% improved symptoms (p=0.09), 76% improved viral clearance (p=0.03), and 1% shorter hospitalization.
Small 72 patient RCT of ivermectin and ivermectin + doxycycline showing faster recovery with ivermectin. The ivermectin + doxycycline group uses only a single dose of ivermectin vs. 5 daily doses for the ivermectin group. PCR testing was only done weekly after day 7, therefore hospitalization time may not match symptomatic recovery. Ivermectin group: 12mg daily for 5 days Ivermectin + doxycycline: 12mg ivermectin single dose independent of mass, 200mg doxycycline + 100mg bid 4 days https://c19p.org/ahmed
11. K. Shimizu, H. Hirata, D. Kabata, N. Tokuhira, M. Koide, A. Ueda, J. Tachino, A. Shintani, A. Uchiyama, Y. Fujino, and H. Ogura, Ivermectin administration is associated with lower gastrointestinal complications and greater ventilator-free days in ventilated patients with COVID-19: A propensity score analysis Dec 2021, J. Infection and Chemotherapy
LATE TREATMENT 88 patient ivermectin late treatment study: 100% lower mortality (p=0.001), 48% lower ventilation (p=0.03), 43% lower ICU admission (p=0.06), and 78% lower progression (p=0.03).
Retrospective 88 ventilated Covid-19 patients in Japan, 39 treated with ivermectin within 3 days of admission, showing significantly reduced incidence of GI complications and mortality, and increased ventilator-free days with treatment. https://c19p.org/shimizu
12. R. Seet, A. Quek, D. Ooi, S. Sengupta, S. Lakshminarasappa, C. Koo, J. So, B. Goh, K. Loh, D. Fisher, H. Teoh, J. Sun, A. Cook, P. Tambyah, and M. Hartman, Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial Apr 2021, Int. J. Infectious Diseases, Volume 106, Page 314-322
1,236 patient ivermectin prophylaxis RCT: 50% fewer symptomatic cases (p=0.0009) and 6% fewer cases (p=0.61).
Prophylaxis RCT in Singapore with 3,037 low-risk patients, showing lower serious cases, lower symptomatic cases, and lower confirmed cases of Covid-19 with all treatments (ivermectin, HCQ, PVP-I, and Zinc + vitamin C) compared to vitamin C. The ivermectin dosage was low for 42 days prophylaxis – only a single dose of 200µg/kg, with a maximum of 12mg. Meta-analysis of vitamin C in 6 previous trials shows a benefit of 16%, so the actual benefit of ivermectin, hydroxychloroquine, and PVP-I may be higher. Cluster RCT with 40 clusters. There were no hospitalizations and no deaths. https://c19p.org/seet
13. S. Lim, C. Hor, K. Tay, A. Mat Jelani, W. Tan, H. Ker, T. Chow, M. Zaid, W. Cheah, H. Lim, K. Khalid, J. Cheng, H. Mohd Unit, N. An, A. Nasruddin, L. Low, S. Khoo, J. Loh, N. Zaidan, S. Ab Wahab, L. Song, H. Koh, T. King, N. Lai, S. Chidambaram, K. Peariasamy, W. Hwong, E. Low, M. Pathmanathan, M. Hamzah, Y. Chan, J. Voo, C. Yap, Y. Chan, L. Vun, K. Kong, Y. Lim, Y. Teoh, A. Abdullah, A. Ramadas, C. Leong, N. Wahab, N. Ismail, I. Ismail, T. Lee, P. Khoo, S. Phua, P. Gopalakrishnan, S. Jaya Selan, I. Ampalakan et al., Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities: The I-TECH Randomized Clinical Trial Nov 2021, JAMA, Volume 182, Issue 4, Page 426
LATE TREATMENT 490 patient ivermectin late treatment RCT: 69% lower mortality (p=0.09), 59% lower ventilation (p=0.17), 22% lower ICU admission (p=0.79), and 31% lower progression (p=0.29).
RCT 490 late stage (>65% lung change chest radiography at baseline) hospitalized patients in Malaysia, showing no significant differences. Mortality was 1.2% for ivermectin vs. 4% for control. If the same event rates continue, the trial would need to add ~13% more patients to reach statistical significance. i.e., by continuing the trial for ~2 weeks, there is a reasonable chance of the result being a statistically significant ~69% reduction in mortality, which would equate to ~4 million lives saved if adopted at the start of the pandemic. The mortality reduction is consistent with the results from all trials to date. While not reaching the significance threshold with the specified test, Bayesian analysis shows a 97% probability that ivermectin reduces mortality. Authors describe the mortality results as “similar” and they are not mentioned in the visual abstract or the conclusion, suggesting substantial investigator bias with a preference for a null hypothesis. https://c19p.org/lim
14. W. Shoumann, A. Hegazy, R. Nafae, M. Ragab, S. Samra, D. Ibrahim, T. Al-Mahrouky, and A. Sileem, Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial Aug 2020, J. Clinical and Diagnostic Research
304 patient ivermectin prophylaxis RCT: 91% fewer symptomatic cases (p=0.001) and 93% lower severe cases (p=0.002).
Pre-exposure prophylaxis trial for asymptomatic close contacts of Covid-19 patients, 203 ivermectin patients and 101 control patients. 7.4% of contacts developed Covid-19 in the ivermectin group vs. 58.4% in the control group. Efficacy for symptomatic cases and severe cases is very similar. Adjusted results are provided only for symptomatic cases. See also: https://c19p.org/shouman
15. N. Okumuş, N. Demirtürk, R. Çetinkaya, R. Güner, İ. Avcı, S. Orhan, P. Konya, B. Şaylan, A. Karalezli, L. Yamanel, B. Kayaaslan, G. Yılmaz, Ü. Savaşçı, F. Eser, and G. Taşkın, Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients Jan 2021, BMC Infectious Diseases, Volume 21, Issue 1
LATE TREATMENT 60 patient ivermectin late treatment RCT: 33% lower mortality (p=0.55), 43% greater improvement (p=0.18), and 80% improved viral clearance (p=0.02).
Small RCT for severe Covid-19 comparing the addition of ivermectin to standard of care (low dose HCQ+AZ+favipiravir), with 30 treatment and 30 control patients in Turkey, showing lower mortality and faster clinical recovery. Authors also investigate the presence of gene mutations that alter ivermectin metabolism, predicting that ivermectin can be used safely without serious side effects in patients without MDR-1/ABCB1 and/or CYP3A4 gene mutation, and recommending monitoring and appropriate treatment if necessary when sequencing is unavailable. https://c19p.org/okumus
16. Ravikirti, R. Roy, C. Pattadar, R. Raj, N. Agarwal, B. Biswas, P. Manjhi, D. Rai, Shyama, A. Kumar, and A. Sarfaraz, Ivermectin as a potential treatment for mild to moderate COVID-19: A double blind randomized placebo-controlled trial Jan 2021, J. Pharmacy & Pharmaceutical Sciences, Volume 24, Page 343-350
EARLY TREATMENT 112 patient ivermectin early treatment RCT: 89% lower mortality (p=0.12), 79% lower ventilation (p=0.1), 14% lower ICU admission (p=0.8), and 89% higher hospital discharge (p=0.12).
RCT with 112 mild and moderate Covid-19 patients in India, showing lower mortality, ventilation, and ICU admission, although not statistically significant due to the small number of events. There was no mortality in the treatment arm (55 patients) versus 7% (4 of 57) in the control arm. The PCR result is subject to confounding by biased loss of follow-up, with 23 lost in the treatment group and 13 in the control group, and 8 more people in the treatment group discharged before day 6. https://c19p.org/ravikirti
17. O. Babalola, C. Bode, A. Ajayi, F. Alakaloko, I. Akase, E. Otrofanowei, O. Salu, W. Adeyemo, A. Ademuyiwa, and S. Omilabu, Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos Jan 2021, QJM: An Int. J. Medicine, Volume 114, Issue 11, Page 780-788
EARLY TREATMENT 60 patient ivermectin early treatment RCT: 64% improved viral clearance (p=0.11) and 41% improved recovery (p=0.07).
Small RCT comparing ivermectin 6mg and 12mg q84hr with lopinavir/ritonavir, showing a statistically significant and dose-dependent effect of ivermectin on reducing the time to PCR-. The study does not report mortality, hospitalization, progression, recovery, etc. The paper does report change in SpO2 (Figure 3, ∆Spo2), where a similar improvement with a smaller p-value is seen with ivermectin; however, this result is unadjusted and there are large differences between groups. Specifically, baseline SpO2 is lower in the control group, giving the control group more room to improve; therefore the actual benefit of ivermectin is likely to be even larger than the benefit in ∆SpO2 shown. See also: https://c19p.org/babalola
18. R. Lima-Morales, P. Méndez-Hernández, Y. Flores, P. Osorno-Romero, C. Sancho-Hernández, E. Cuecuecha-Rugerio, A. Nava-Zamora, D. Hernández-Galdamez, D. Romo-Dueñas, and J. Salmerón, Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico Feb 2021, Int. J. Infectious Diseases, Volume 105, Page 598-605
LATE TREATMENT 768 patient ivermectin late treatment study: 78% lower mortality (p=0.001), 52% lower ventilation (p=0.15), 67% lower hospitalization (p=0.001), and 59% improved recovery (p=0.001).
Prospective trial of 768 Covid-19 outpatients in Mexico, 481 treated with ivermectin, AZ, montelukast, and aspirin, and 287 control patients with various treatments, showing significantly lower mortality and hospitalization, and significantly higher recovery at 14 days with treatment. https://c19p.org/limamorales
19. M. Mayer, A. Krolewiecki, A. Ferrero, M. Bocchio, J. Barbero, M. Miguel, A. Paladini, C. Delgado, J. Ojeda, C. Elorza, A. Bertone, P. Fleitas, G. Vera, and M. Kohan, Safety and Efficacy of a MEURI Program for the Use of High Dose Ivermectin in COVID-19 PatientsSep 2021, Frontiers in Public Health, Volume 10
EARLY TREATMENT 21,232 patient ivermectin early treatment study: 55% lower mortality (p<0.0001) and 66% lower ICU admission (p<0.0001).
Retrospective 21,232 patients in Argentina, 3,266 assigned to ivermectin treatment, showing lower mortality with treatment. Greater benefits were seen for patients >40, and a dose-dependent response was found. https://c19p.org/mayer
20. I. De Jesús Ascencio-Montiel, J. Tomás-López, V. Álvarez-Medina, L. Gil-Velázquez, H. Vega-Vega, H. Vargas-Sánchez, M. Cervantes-Ocampo, M. Villasís-Keever, C. González-Bonilla, and C. Duque-Molina, A Multimodal Strategy to Reduce the Risk of Hospitalization/death in Ambulatory Patients with COVID-19 Jan 2022, Archives of Medical Research
EARLY TREATMENT 28,048 patient ivermectin early treatment study: 59% lower combined mortality/hospitalization (p<0.0001), 15% lower mortality (p=0.16), 9% lower ventilation (p=0.51), and 48% lower hospitalization (p<0.0001).
Retrospective 28,048 Covid+ patients in Mexico, 7,898 receiving a treatment kit including low-dose ivermectin, AZ, aspirin, and acetaminophen, shower lower mortality/hospitalization for those receiving the kit. Delivery of the treatment kit was based on availability in the medical units. Adherence is unknown and may be low. Adjusted results are only provided for combined mortality/hospitalization. https://c19p.org/dejesusascenciomontiel
21. Z. Aref, S. Bazeed, M. Hassan, A. Hassan, A. Ghweil, M. Sayed, A. Rashad, H. Mansour, and A. Abdelmaksoud, Possible Role of Ivermectin Mucoadhesive Nanosuspension Nasal Spray in Recovery of Post-COVID-19 Anosmia Sep 2022, Infection and Drug Resistance, Volume Volume 15, Page 5483-5494
LATE TREATMENT 96 patient ivermectin long Covid RCT: 74% faster recovery (p=0.0005).
96 patient RCT showing faster resolution of post-Covid anosmia with an ivermectin nanosuspension nasal spray. https://c19p.org/aref2
22. A. Mohan, P. Tiwari, T. Suri, S. Mittal, A. Patel, A. Jain, T. Velpandian, U. Das, T. Boppana, R. Pandey, S. Shelke, A. Singh, S. Bhatnagar, S. Masih, S. Mahajan, T. Dwivedi, B. Sahoo, A. Pandit, S. Bhopale, S. Vig, R. Gupta, K. Madan, V. Hadda, N. Gupta, R. Garg, V. Meena, and R. Guleria, Single-dose oral ivermectin in mild and moderate COVID-19 (RIVET-COV): a single-centre randomized, placebo-controlled trial Feb 2021, J. Infection and Chemotherapy, Volume 27, Issue 12, Page 1743-1749
EARLY TREATMENT 157 patient ivermectin early treatment RCT: 62% improved recovery (p=0.27) and 24% improved viral clearance (p=0.18).
RCT in India with low-risk patients, comparing 24mg ivermectin, 12mg ivermectin, and placebo showing non-statistically significant improvements in recovery and PCR+ status (day 5 both arms, day 7 24mg only) with treatment, and showing greater improvement for the higher dose arm. Viral load decline was similar in all arms – absolute values are lower for ivermectin in a dose-dependent manner; however the baseline value for the ivermectin groups was lower, leaving less room for change. There were no deaths or use of mechanical ventilation. There were no serious adverse events. Pre-specified protocol prioritizes clinical outcomes over PCR results. https://c19p.org/mohan
23. R. Faisal, S. Shah, and M. Hussain, Potential use of azithromycin alone and in combination with ivermectin in fighting against the symptoms of COVID-19 May 2021, The Professional Medical J., Volume 28, Issue 05, Page 737-741
EARLY TREATMENT 100 patient ivermectin early treatment RCT: 68% improved recovery (p=0.005).
RCT 100 outpatients in Pakistan, 50 treated with ivermectin, showing faster recovery with ivermectin. All patients received AZ, zinc, vitamin C, vitamin D, and acetaminophen. Details of randomization were not provided. No mortality or hospitalization was reported. https://c19p.org/faisal
24. B. George, M. Moorthy, U. Kulkarni, S. Selvarajan, P. Rupali, D. Christopher, T. Balamugesh, W. Rose, K. Lakshmi, A. Devasia, N. Fouzia, A. Korula, S. Lionel, A. Abraham, and V. Mathews, Single Dose of Ivermectin is not Useful in Patients with Hematological Disorders and COVID-19 Illness: A Phase II B Open Labelled Randomized Controlled TrialMay 2022, Indian J. Hematology and Blood Transfusion
LATE TREATMENT 112 patient ivermectin late treatment RCT: 30% lower mortality (p=0.55), 19% faster recovery (p=0.37), 33% lower progression (p=0.41), and 33% worse viral clearance (p=0.5).
RCT with 35 single dose 24mg, 38 single dose 12mg, and 39 standard of care hospitalized patients with hematological illnesses in India, showing no significant differences. Results were better for 24mg vs. 12mg for all symptomatic outcomes. Viral clearance results do not follow the randomization with less than 50% of patients tested at day 7, and no adjusted results are provided. Results were obtained for only 43.8% of ivermectin patients and 56.4% of control patients at day 7 and may not be comparable due to the large difference in the percentage of patients tested. Lower test coverage in the ivermectin group is likely related to faster recovery. https://c19p.org/george
25. A. Manomaipiboon, K. Pholtawornkulchai, S. Poopipatpab, S. Suraamornkul, J. Maneerit, W. Ruksakul, U. Phumisantiphong, and T. Trakarnvanich, Efficacy and safety of ivermectin in the treatment of mild-to-moderate COVID-19 infection: A randomized, double blind, placebo, controlled trial Feb 2022, Trials, Volume 23, Issue 1
EARLY TREATMENT 72 patient ivermectin early treatment RCT: 43% improved recovery (p=0.26) and 5% improved viral clearance (p=1).
Small RCT with 72 low-risk patients in Thailand, showing improved recovery with ivermectin, without statistical significance. All patients recovered and there was no escalation of care in either group. There were no adverse events. https://c19p.org/manomaipiboon
26. H. Hashim, M. Maulood, C. Ali, A. Rasheed, D. Fatak, K. Kabah, A. Abdulamir, Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq Oct 2020, Iraqi J. Medical Science
LATE TREATMENT 140 patient ivermectin late treatment RCT: 92% lower mortality (p=0.03), 83% lower progression (p=0.07), and 41% faster recovery (p=0.0001).
RCT 70 ivermectin+doxycycline patients and 70 control patients showing reduced time to recovery and reduced mortality with treatment. Earlier treatment was more successful. For ethical reasons, critical patients were all in the treatment group. NCT04591600. https://c19p.org/hashim
27. A. Mirahmadizadeh, A. Semati, A. Heiran, M. Ebrahimi, A. Hemmati, M. Karimi, S. Basir, M. Zare, A. Charlys da Costa, M. Zeinali, M. Sargolzaee, and O. Eilami, Efficacy of single-dose and double-dose ivermectin early treatment in preventing progression to hospitalization in mild COVID-19: A multi-arm, parallel-group randomized, double-blind, placebo-controlled trial Jun 2022, Respirology
EARLY TREATMENT 261 patient ivermectin early treatment RCT: 67% lower ventilation (p=0.37), 46% lower hospitalization (p=0.22), and 39% improved recovery (p=0.27).
RCT with 131 24mg ivermectin, 130 12mg ivermectin, and 130 placebo patients, showing no significant differences in outcomes. Lower ventilation and hospitalization was seen with treatment, in a dose-dependent manner, but not reaching statistical significance with the small number of events. https://c19p.org/mirahmadizadeh
28. I. Efimenko, S. Nackeeran, S. Jabori, J. Zamora, S. Danker, and D. Singh, Treatment with Ivermectin Is Associated with Decreased Mortality in COVID-19 Patients: Analysis of a National Federated Database Feb 2022, Int. J. Infectious Diseases, Volume 116, Page S40
SELF-CENSORED LATE TREATMENT 41,608 patient ivermectin late treatment propensity score matching study: 69% lower mortality (p<0.0001).
Retrospective 41,608 patients in the US, 1,072 treated with ivermectin and 40,536 treated with remdesivir, showing lower mortality with ivermectin treatment. This study was presented at a conference (IMED 2021). Submissions were peer-reviewed. The treatment/control group sizes align with the estimated percentage of hospitals that used ivermectin vs. remdesivir. Hospitals in the US received financial incentives to use remdesivir. Authors have self-censored the conference report of this result, not due to any error in the analysis, but because they believe ivermectin “has proven to be ineffective in clinical trials.” This is incorrect, while some studies show no statistically significant effect, studies show statistically significant positive results for one or more outcomes (prospective and retrospective studies, including RCTs). The self-censorship and decision not to submit to a journal provide further evidence of a negative publication bias for ivermectin research. https://c19p.org/efimenko
29. S. Mondal, A. Singha, D. Das, S. Neogi, P. Gargari, M. Shah, D. Arjunan, P. Mukhopadhyay, S. Ghosh, J. Chowdhury, S. Chowdhury, Prevalence of COVID-19 Infection and Identification of Risk Factors among Asymptomatic Healthcare Workers: A Serosurvey Involving Multiple Hospitals in West Bengal May 2021, J. of the Indian Medical Association
1,470 patient ivermectin prophylaxis study: 88% fewer symptomatic cases (p=0.006).
Retrospective 1,470 healthcare workers in India, showing significantly lower risk of symptomatic Covid-19 with ivermectin prophylaxis. https://c19p.org/mondal
30. S. Mourya, A. Thakur, D. Hada, V. Kulshreshtha, Y. Sharma, Comparative Analytical Study of Two Different Drug Regimens in Treatment of Covid 19 Positive Patients in Index Medical College Hospital and Research Center, Indore, India Mar 2021, Int. J. Health and Clinical Research
EARLY TREATMENT 100 patient ivermectin early treatment study: 89% improved viral clearance (p<0.0001).
Retrospective 100 patients in India with 50 treated with ivermectin, and standard of care for all patients including HCQ+AZ, showing much higher viral clearance with ivermectin. Baseline clinical status was worse in the control group. Time of testing after treatment initiation was longer in the control group (mean 7.24 days versus 5.22 days). https://c19p.org/mourya
31. P. Behera, B. Patro, B. Padhy, P. Mohapatra, S. Bal, P. Chandanshive, R. Mohanty, S. Ravikumar, A. Singh, S. Singh, S. Pentapati, J. Nair, and G. Batmanbane, Prophylactic Role of Ivermectin in Severe Acute Respiratory Syndrome Coronavirus 2 Infection Among Healthcare Workers Feb 2021, Cureus 13:8
3,346 patient ivermectin prophylaxis study: 83% fewer cases (p=0.001).
Prospective prophylaxis study with 3,532 healthcare workers, 2,199 receiving two-dose ivermectin prophylaxis, showing adjusted relative risk of confirmed Covid-19 with treatment 0.17 [0.12-0.23] p<0.001. 186 patients took only the first dose, and no significant difference was observed for this group. The same group published an earlier small study with 117 ivermectin patients. There were no serious adverse events. https://c19p.org/behera2
32. M. Alam, R. Murshed, P. Gomes, Z. Masud, S. Saber, M. Chaklader, F. Khanam, M. Hossain, A. Momen, N. Yasmin, R. Alam, A. Sultana, and R. Robin, Ivermectin as Pre-exposure Prophylaxis for COVID-19 among Healthcare Providers in a Selected Tertiary Hospital in Dhaka – An Observational Study Dec 2020, European J. Medical and Health Sciences, Volume 2, Issue 6
118 patient ivermectin prophylaxis study: 91% fewer cases (p<0.0001).
91% reduction in Covid-19 cases with ivermectin prophylaxis. 118 healthcare workers in Bangladesh, 58 receiving ivermectin 12mg monthly, showing RR 0.094, p < 0.0001. https://c19p.org/alam2
33. P. Behera, B. Patro, A. Singh, P. Chandanshive, R. S. R., S. Pradhan, S. Pentapati, G. Batmanabane, P. Mohapatra, B. Padhy, S. Bal, S. Singh, and R. Mohanty, Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study Nov 2020, PLoS ONE, Volume 16, Issue 2, Page e0247163
372 patient ivermectin prophylaxis study: 54% fewer cases (p=0.0007).
Retrospective matched case-control prophylaxis study for hydroxychloroquine, ivermectin, and vitamin C with 372 healthcare workers, showing lower Covid-19 incidence for all treatments, with statistical significance reached for ivermectin. Hydroxychloroquine OR 0.56, p = 0.29 Ivermectin OR 0.27, p < 0.001 Vitamin C OR 0.82, p = 0.58 https://c19p.org/beherai
34. J. Rajter, M. Sherman, N. Fatteh, F. Vogel, J. Sacks, and J. Rajter, Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study) Oct 2020, Chest, Volume 159, Issue 1, Page 85-92
LATE TREATMENT 280 patient ivermectin late treatment propensity score matching study: 46% lower mortality (p=0.05) and 64% lower ventilation (p=0.1).
Retrospective 280 hospitalized patients showing lower mortality with ivermectin (13.3% vs 24.5%), propensity matched odds ratio 0.47 [0.22-0.99], p=0.045. https://c19p.org/rajter
35. J. Morgenstern, J. Redondo, A. Olavarria, I. Rondon, S. Roca, A. De Leon, J. Canela, J. Tavares, M. Minaya, O. Lopez, A. Castillo, A. Placido, R. Cruz, Y. Merette, M. Toribio, and J. Francisco, Ivermectin as a SARS-CoV-2 Pre-Exposure Prophylaxis Method in Healthcare Workers: A Propensity Score-Matched Retrospective Cohort Study Apr 2021, Cureus
542 patient ivermectin prophylaxis propensity score matching study: 74% fewer cases (p=0.008).
Propensity matched retrospective prophylaxis study of healthcare workers in the Dominican Republic showing significantly lower cases with treatment, and no hospitalization with treatment (versus 2 in the propensity score matching matched control group). The cases with treatment were mostly in the first week, with only one case in the second and third weeks, and none in the fourth week. There were no severe side effects. In post-hoc analysis, as the treatment group discontinued treatment over time, their protection also decreased. https://c19p.org/morgenstern2
36. K. Shah Bukhari, A. Asghar, N. Perveen, A. Hayat, S. Mangat, K. Butt, M. Abdullah, T. Fatima, A. Mustafa, and T. Iqbal, Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease Jan 2021, medRxiv
EARLY TREATMENT 86 patient ivermectin early treatment RCT: 82% improved viral clearance (p<0.0001).
RCT of relatively low-risk hospitalized patients with 50 ivermectin and 50 control patients showing significantly faster viral clearance with treatment. Nine patients in the treatment arm were lost to follow-up compared with 5 in the control arm, which could be in part due to faster recovery with treatment. There were no safety concerns. No mortality was reported. The numbers in Table 3 are the number of patients that became negative on that day, i.e., non-cumulative. Standard of care included vitamin C and vitamin D. NCT04392713. https://c19p.org/bukhari
37. G. Espitia-Hernandez, L. Munguia, D. Diaz-Chiguer, R. Lopez-Elizalde, F. Jimenez-Ponce, Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study Aug 2020, Biomedical Research
EARLY TREATMENT 35 patient ivermectin early treatment study: 70% faster recovery (p=0.0001) and 97% improved viral clearance (p<0.0001).
Small study with 28 patients treated with ivermectin + AZ + cholecalciferol and 7 control patients. All treated patients were PCR- at day 10 while all control patients remained PCR+. The mean duration of symptoms was 3 days in the treatment group and 10 days in the control group. https://c19p.org/espitiahernandez
38. J. Merino, V. Borja, O. López, J. Ochoa, E. Clark, L. Petersen, S. Caballero, Ivermectin and the odds of hospitalization due to COVID-19: evidence from a quasi-experimental analysis based on a public intervention in Mexico CityMay 2021, Preprint
CENSORED EARLY TREATMENT 77,381 patient ivermectin early treatment study: 74% lower hospitalization (p=0.001).
Analysis of Mexico City’s use of an ivermectin-based medical kit, showing significantly lower hospitalization with use. Authors use logistic-regression models with matched observations, including adjustments for age, sex, Covid severity, and comorbidities. This preprint was censored by the original preprint host.Censors claim that the government treatment program, which used approved medications and saved over 500 people from hospitalization, was unethical. In part they also indicate that studies of “the effects of a medication on a disease outcome” are outside the scope of their site; however, retroactively censoring a paper for this reason is not appropriate. The author’s response (not provided by the censors) can be found here: Authors provide the data and code for the study, and the results have been independently verified. https://c19p.org/merino
39. C. Bernigaud, D. Guillemot, A. Ahmed-Belkacem, L. Grimaldi-Bensouda, A. Lespine, F. Berry, L. Softic, C. Chenost, G. Do-Pham, B. Giraudeau, S. Fourati, and O. Chosidow, Ivermectin benefit: from scabies to COVID-19, an example of serendipity Nov 2020, Annals of Dermatology and Venereology, Volume 147, Issue 12, Page A194
3,131 patient ivermectin prophylaxis study: 99% lower mortality (p=0.08) and 55% fewer cases (p=0.01).
69 residents of a French care home, median age 90, were treated with ivermectin for a scabies outbreak. 3,062 residents in 45 nearby comparable homes were used as controls. Seven of 69 treated patients had probable or certain Covid-19, with no serious cases and no deaths. In comparable care homes in the same district, matched by age and socio-economic level, there was 22.6% Covid-19 and 5% death. https://c19p.org/bernigaud
40. M. Ozer, S. Goksu, R. Conception, E. Ulker, R. Balderas, M. Mahdi, Z. Manning, K. To, M. Effendi, R. Anandakrishnan, M. Whitman, and M. Gugnani, Effectiveness and Safety of Ivermectin in COVID-19 Patients: A Prospective Study at A Safety-Net Hospital Nov 2021, J. Medical Virology
LATE TREATMENT 120 patient ivermectin late treatment study: 75% lower mortality (p=0.09), 13% lower ventilation (p=0.2), and 9% longer hospitalization (p=0.09).
Small prospective propensity score matching study in the US, showing 75% lower mortality with ivermectin treatment, without reaching statistical significance, significantly shorter ventilation and ICU time, and longer hospitalization time. Authors leave the statistically significant improvements in ventilation and ICU time out of the abstract and conclusions, and incorrectly state that there were no differences in other outcomes. Authors are ambiguous on the primary outcome, referring to the primary mortality outcome in one case, and “clinical outcomes, measured by the rate of intubation, length of hospital stay, and mechanical ventilation duration” in another case. The longer hospitalization time may be partially due to the greater mortality in the control group. https://c19p.org/ozer
41. F. Ochoa-Jaramillo, F. Rodriguez-Vega, N. Cardona-Castro, V. Posada-Velez, D. Rojas-Gualdron, H. Contreras-Martinez, A. Romero-Millan, and J. Porras-Mansilla, Clinical efficacy and safety of ivermectin (400 μg/kg, single dose) in patients with severe COVID-19: a randomized clinical trial Oct 2022, Revista Infectio
LATE TREATMENT 75 patient ivermectin late treatment RCT: 57% lower mortality (p=0.35), 34% higher ventilation (p=0.62), and 37% higher ICU admission (p=0.52).
RCT 75 very late stage inpatients in Colombia, showing no significant difference in outcomes with a single dose of 400μg/kg ivermectin. https://c19p.org/ochoajaramillo
42. L. Pierre and F. Christine, Ivermectin and COVID-19 in Care Home: Case Report Apr 2021, J. Infectious Diseases and Epidemiology, Volume 7, Issue 4
EARLY TREATMENT 25 patient ivermectin early treatment study: 70% lower mortality (p=0.34) and 55% lower severe cases (p=0.11).
Small quasi-randomized (patient choice) study with 25 PCR+ patients in a nursing home offered ivermectin, of which 10 chose to be treated. The mean age was 83.5 in the treatment group and 81.8 in the control group. There was lower mortality and fewer serious cases with treatment. https://c19p.org/loue
43. L. Kerr, F. Cadegiani, F. Baldi, R. Lobo, W. Assagra, F. Proença, P. Kory, J. Hibberd, and J. Chamie-Quintero, Ivermectin Prophylaxis Used for COVID-19: A Citywide, Prospective, Observational Study of 223,128 Subjects Using Propensity Score Matching Dec 2021, Cureus
159,561 patient ivermectin prophylaxis propensity score matching study: 70% lower mortality (p<0.0001), 67% lower hospitalization (p<0.0001), and 44% fewer cases (p<0.0001).
Propensity score matching retrospective 220,517 patients in Brazil,133,051 taking ivermectin as part of a citywide prophylaxis program, showing significantly lower hospitalization and mortality with treatment. Additional results are presented in a 90-minute video presentation hosted by FLCCC here, including improved efficacy with analysis based on irregular/regular use, and a strong dose-response relationship. https://c19p.org/kerr
44. E. Osati, G. Shayo, T. Nagu, R. Sangeda, C. Moshiro, L. Vumilia, L. Samwel, P. Mhame, M. Nkya, D. Rainer, M. John, C. Mbije, G. Nyaisonga, K. Kilonzo, M. Nicholaus, J. Seni, A. Muniko, B. Wajanga, A. Ramadhani, N. Adams, S. Shekalaghe, and A. Makubi, Clinical manifestations and mortality among hospitalized COVID-19 patients in Tanzania, 2021-2022. Jul 2023, medRxiv
LATE TREATMENT 1,387 patient ivermectin late treatment study: 32% lower mortality (p=0.02).
Retrospective 1,387 hospitalized PCR confirmed Covid-19 patients in Tanzania, showing lower mortality with ivermectin treatment and with steroid treatment in multivariable analysis. https://c19p.org/osati
45. H. Elalfy, T. Besheer, A. El‐Mesery, A. El‐Gilany, M. Soliman, A. Alhawarey, M. Alegezy, T. Elhadidy, A. Hewidy, H. Zaghloul, M. Neamatallah, D. Raafat, W. El‐Emshaty, N. Abo El Kheir, and M. El‐Bendary, Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-1 Feb 2021, J. Med. Virol., Volume 93, Issue 5, Page 3176-3183
EARLY TREATMENT 113 patient ivermectin early treatment study: 87% improved viral clearance (p<0.0001).
Non-randomized controlled trial with 62 mild and early moderate patients with home treatment with ivermectin + nitazoxanide + ribavirin + zinc, showing significantly faster viral clearance. https://c19p.org/elalfy
46. Huvemek et al., Kovid-19 – Huvemek® Phase 2 clinical trial Mar 2021, Huvemek, Press Release
LATE TREATMENT 100 patient ivermectin late treatment RCT: 32% greater improvement (p=0.28).
Multicenter double-blind RCT with 100 hospitalized patients in Bulgaria showing faster viral clearance, greater clinical improvement, and improved biomarkers with treatment. Limited data has been reported currently. No serious adverse events were observed. https://c19p.org/petkov
47. V. Spoorthi, S. Sasank, Utility of Ivermectin and Doxycycline combination for the treatment of SARSCoV-2 Nov 2020, IAIM, 2020, 177-182
LATE TREATMENT 100 patient ivermectin late treatment study: 21% faster recovery (p=0.03) and 16% shorter hospitalization (p=0.01).
100 patient prospective trial of ivermectin + doxycycline showing reduced time to symptom resolution and shorter hospital stay with treatment. https://c19p.org/spoorthi
48. M. Rezai, F. Ahangarkani, A. Hill, L. Ellis, M. Mirchandani, A. Davoudi, G. Eslami, F. Roozbeh, F. Babamahmoodi, N. Rouhani, A. Alikhani, N. Najafi, R. Ghasemian, H. Mehravaran, A. Hajialibeig, M. Navaeifar, L. Shahbaznejad, G. Rahimzadeh, M. Saeedi, R. Alizadeh-Navai, M. Moosazadeh, S. Saeedi, S. Razavi-Amoli, S. Rezai, F. Rostami-Maskopaee, F. Hosseinzadeh, F. Movahedi, J. Markowitz, and R. Valadan, Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials Jun 2022, Frontiers in Medicine, Volume 9
LATE TREATMENT 609 patient ivermectin late treatment RCT: 31% lower mortality (p=0.36), 50% lower ventilation (p=0.07), 16% lower ICU admission (p=0.47), and 11% longer hospitalization (p=0.009).
RCT 609 inpatients in Iran. Reported outcomes are very different from the pre-specified outcomes. Dose was limited at a maximum of 30mg for 75+kg, resulting in underdosing for patients at higher risk. Almost all patients received remdesivir (which has a significant independent safety profile), most patients received famotidine and vitamin C, and many patients received vitamin D, metformin, and zinc, limiting room for improvement. 32% of patients were lost to follow-up. All negative outcomes are protocol violations and are not listed in the protocol, including the novel “relative recovery” outcome. Authors include a researcher caught on video admitting that conclusions on ivermectin research were influenced by a funder. https://c19p.org/rezai2
49. T. Siripongboonsitti, K. Tawinprai, P. Avirutnan, K. Jitobaom, and P. Auewarakul, A Randomized Trial to Assess the Acceleration of Viral Clearance by the Combination Favipiravir/Ivermectin/Niclosamide in Mild-to-Moderate COVID-19 Adult Patients (FINCOV)Mar 2024, J. Infection and Public Health, Volume 17, Issue 5, Page 897-905
EARLY TREATMENT 60 patient ivermectin early treatment RCT: 39% improved recovery (p=0.19) and 6% improved viral clearance (p=0.75).
RCT 60 low-risk outpatients, median age 31, with mild to moderate Covid-19 showing no significant differences with combined favipiravir/ivermectin/niclosamide treatment compared to favipiravir alone. There was limited room for improvement with almost no progression and no hospitalization, ICU admission, supplemental oxygen, or mortality. The combined group showed significantly improved visual analog scale scores for cough, runny nose, and diarrhea from day 3. Authors note that “the WHO-CPS were significantly decreased among FPV/IVM/NCL vs FPV alone on day 10”; however the degree of improvement cannot be determined based on the values reported. Authors state that “All data generated or analyzed during this study are included in this published article,” which is incorrect – only summary statistics are published. The trial registration states that data will not be made available. This raises concerns, especially given many inconsistencies in the published data: https://c19p.org/siripongboonsitti6
50. S. Budhiraja, A. Soni, V. Jha, A. Indrayan, A. Dewan, O. Singh, Y. Singh, I. Chugh, V. Arora, R. Pande, A. Ansari, and S. Jha, Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience Nov 2020, medRxiv
LATE TREATMENT 976 patient ivermectin late treatment study: 99% lower mortality (p=0.04).
Retrospective 976 hospitalized patients with 34 treated with ivermectin showing lower mortality with ivermectin in unadjusted results. https://c19p.org/budhirajai
51. J. Llenas-García, A. Del Pozo, A. Talaya, N. Roig-Sánchez, N. Poveda Ruiz, C. Devesa García, E. Borrajo Brunete, I. González Cuello, A. Lucas Dato, M. Navarro, and P. Wikman-Jorgensen, Ivermectin Effect on In-Hospital Mortality and Need for Respiratory Support in COVID-19 Pneumonia: Propensity Score-Matched Retrospective Study May 2023, Viruses, Volume 15, Issue 5, Page 1138
LATE TREATMENT 192 patient ivermectin late treatment study: 17% lower mortality (p=0.82), 18% lower need for oxygen therapy (p=0.37), 23% lower progression (p=0.52), and 4% higher ICU admission (p=0.92).
Retrospective 96 late stage patients receiving a single dose of 200 μg/kg ivermectin(inappropriate dose for strongyloides or Covid) and 96 matched controls, showing no significant difference in outcomes. Authors note that this may be due to the low dose used, so late treatment and low dose contributed to these findings. https://c19p.org/llenasgarcia
52. S. Baguma, C. Okot, N. Onira, P. Apiyo, D. Acullu, P. Layet, J. Oloya, D. Ochula, P. Atim, P. Olwedo, F. Pebolo, F. Oyat, J. Oola, J. Aloyo, E. Ikoona, and D. Kitara, Characteristics of the COVID-19 patients treated at Gulu Regional Referral Hospital, Northern Uganda: A cross-sectional study Dec 2021, Research Square
LATE TREATMENT 481 patient ivermectin late treatment study: 97% lower mortality (p=0.31).
Retrospective Covid+ hospitalized patients in Uganda, showing no statistically significant difference in mortality with ivermectin; however, only 7 of the 481 patients received ivermectin. https://c19p.org/baguma
53. W. Schilling, P. Jittamala, J. Watson, M. Ekkapongpisit, T. Siripoon, T. Ngamprasertchai, V. Luvira, S. Pongwilai, C. Cruz, J. Callery, S. Boyd, V. Kruabkontho, T. Ngernseng, J. Tubprasert, M. Abdad, N. Piaraksa, K. Suwannasin, P. Hanboonkunupakarn, B. Hanboonkunupakarn, S. Sookprome, K. Poovorawan, J. Thaipadungpanit, S. Blacksell, M. Imwong, J. Tarning, W. Taylor, V. Chotivanich, C. Sangketchon, W. Ruksakul, K. Chotivanich, M. Teixeira, S. Pukrittayakamee, A. Dondorp, N. Day, W. Piyaphanee, W. Phumratanaprapin, and N. White, Pharmacometrics of high dose ivermectin in early COVID-19: an open label, randomized, controlled adaptive platform trial (PLATCOV)Jul 2022, eLife, Volume 12
EARLY TREATMENT 90 patient ivermectin early treatment RCT: 86% lower progression (p=0.24) and 9% worse viral clearance (p=0.36).
Very high conflict of interest RCT with design optimized for a null result: very low-risk patients, high existing immunity, post-hoc change to exclude patients more likely to benefit. There was no significant difference in viral clearance among low-risk patients with high viral load at baseline. All 3 progression events occurred in the control arm – one hospitalization and two cases of Covid-19 related rhabdomyolysis. Patients in both arms cleared the virus quickly with a viral clearance half-life of 21.1 hours vs. 19.2 hours, which may be in part due to prior immunity. With rapid viral clearance and very low-risk patients, infection is less likely to spread to other tissues. Systemic treatment is less applicable, and has less time to reach therapeutic concentrations before self-recovery. Treatment administered directly to the respiratory tract, with some data showing that may be more effective for Covid-19. https://c19p.org/schilling
54. K. Abbas, S. Muhammad, and S. Ding, The Effect of Ivermectin on Reducing Viral Symptoms in Patients with Mild COVID-19 Dec 2021, Indian J. Pharmaceutical Sciences, Volume 84, Issue S1
EARLY TREATMENT 202 patient ivermectin early treatment RCT: 41% lower progression (p=0.54) and 36% improved recovery (p=0.04).
RCT 99 ivermectin and 103 control low-risk patients in China, up to 7 days from symptom onset, showing statistically significant improvement in recovery with treatment, and non-statistically significant improvements in recovery time and deterioration. Authors selectively omitted the p-value for recovery which shows statistical significance. Very little information on the patients is provided (only age, gender, and insurance status). The table, text, and abstract show three different versions of recovery numbers. The table and abstract show two different versions of recovery time. The abstract contains a hazard ratio that is not in the text, and no statistical methods are reported. Given the selective omission of the statistically significant recovery p-value, three different sets of numbers for that outcome, and other inconsistencies, the data in this study does not appear to be very reliable. Patients >50 were excluded. https://c19p.org/abbas2
55. T. Wada, M. Hibino, H. Aono, S. Kyoda, Y. Iwadate, E. Shishido, K. Ikeda, N. Kinoshita, Y. Matsuda, S. Otani, R. Kameda, K. Matoba, M. Nonaka, M. Maeda, Y. Kumagai, J. Ako, M. Shichiri, K. Naoki, M. Katagiri, M. Takaso, M. Iwamura, K. Katayama, T. Miyatsuka, Y. Orihashi, and K. Yamaoka, Efficacy and safety of single-dose ivermectin in mild-to-moderate COVID-19: the double-blind, randomized, placebo-controlled CORVETTE-01 trial May 2023, Frontiers in Medicine, Volume 10
LATE TREATMENT 214 patient ivermectin late treatment RCT: 19% lower progression (p=0.46), 14% higher need for oxygen therapy (p=0.46), 23% worse improvement (p=0.61), and 60% improved recovery (p=0.17).
Late treatment (6.6 days after onset/PCR+) RCT with 221 low-risk (no deaths) Covid-19 patients in Japan, showing no significant difference in viral clearance with a single dose of ivermectin under fasting. Authors note that a single 200 μg/kg dose under fasting was used as approved in Japan, and that that verylow-dose, single-day dosing, and fasting administration (~2.5 times lower plasma concentration) limit applicability, and that studies with more favorable outcomes generally used a higher dose or multi-day dosing. Details of PCR testing are not provided but the very slow clearance within the low-risk population suggests a very high concentration/time value that may not accurately represent any reduction in replication-competent viral load. An erratum notes a conflict of interest for a reviewer that was a Merck employee.https://c19p.org/wada
56. Y. Thairu, O. Babalola, A. Ajayi, Y. Ndanusa, J. Ogedengbe, and O. O., A Comparison of Ivermectin and Non Ivermectin Based Regimen for COVID-19 in Abuja: Effects on Virus Clearance, Days-to-discharge and Mortality Feb 2022, J. Pharmaceutical Research Int., Page 1-19
LATE TREATMENT 87 patient ivermectin late treatment propensity score matching study: 88% lower mortality (p=0.12), 55% higher hospital discharge (p=0.0001), and 95% improved viral clearance (p=0.001).
Retrospective 87 patients in Nigeria, 61 treated with ivermectin, showing lower mortality, faster recovery, and faster viral clearance with ivermectin treatment. All patients received zinc and vitamin C. A synergistic effect was seen for viral clearance when ivermectin and remdesivir were combined. Subject to confounding by time (possible difference in Covid variant), with ivermectin patients from April-June 2021, and non-ivermectin patients from September-November 2021. https://c19p.org/thairu
57. N. Rezk, A. Elsayed Sileem, D. Gad, and A. Khalil, miRNA-223-3p, miRNA- 2909 and Cytokines Expression in COVID-19 Patients Treated with Ivermectin Oct 2021, Zagazig University Medical J., Volume 0, Issue 0, Page 0-0
LATE TREATMENT 320 patient ivermectin late treatment study: 56% lower progression (p=0.06), 33% improved recovery (p=0.27), and 27% faster viral clearance (p=0.01).
Prospective 320 hospitalized moderate Covid-19+ patients in Egypt, 160 treated with ivermectin, showing lower mortality, improved recovery, and decreased cytokine expression with treatment. All patients were treated with hydroxychloroquine. https://c19p.org/rezk
58. R. Qadeer, S. Kashif, D. Kumar, M. Mehmmood, J. Lal, and F. ., Ivermectin A Potential Treatment In Covid-19, Related to Critical Illness Aug 2022, Pakistan J. Medical and Health Sciences, Volume 16, Issue 8, Page 24-26
LATE TREATMENT 210 patient ivermectin late treatment study: 58% improved viral clearance (p<0.0001).
Prospective convenience sampling study of 210 hospitalized age-matched Covid-19 patients, showing faster viral clearance with ivermectin. Baseline information per group is not provided. https://c19p.org/qadeer
59. S. Samajdar, S. Mukherjee, T. Mandal, J. Paul, Ivermectin and Hydroxychloroquine for Chemo-Prophylaxis of COVID-19: A Questionnaire Survey of Perception and Prescribing Practice of Physicians vis-a-vis Outcomes Nov 2021, J. the Association of Physicians India
309 patient ivermectin prophylaxis study: 80% fewer cases (p<0.0001).
Physician survey in India with 164 ivermectin prophylaxis, 129 hydroxychloroquine prophylaxis, and 81 control patients, showing significantly lower Covid-19 cases with treatment. Details of the treatment and control groups and the definition of cases are not provided, and the results are subject to survey bias. Authors also report on community prophylaxis but present only combined ivermectin/ hydroxychloroquine results. https://c19p.org/samajdar
60. M. Mukarram, Ivermectin Use Associated with Reduced Duration of Covid-19 Febrile Illness in a Community Setting Dec 2020, Int. J. Clinical Studies & Medical Case Reports, Volume 13, Issue 4
EARLY TREATMENT 90 patient ivermectin early treatment study: 92% improved recovery (p=0.04).
Retrospective 95 outpatients in Pakistan with strong clinical suspicion of Covid-19 (testing was not widely available), with 40 patients treated with ivermectin, showing significantly shorter duration of febrile illness with treatment. Most patients also received HCQ, AZ, zinc, and aspirin. Authors note that there was a treatment delay-response relationship. https://c19p.org/ghauri
61. M. Hellwig and A. Maia, A COVID-19 Prophylaxis? Lower incidence associated with prophylactic administration of Ivermectin Nov 2020, Int. J. Antimicrobial Agents, Volume 57, Issue 1, Page 106248
Ivermectin prophylaxis study: 78% fewer cases (p=0.02).
Analysis of Covid-19 cases vs. widespread prophylactic use of ivermectin for parasitic infections showing significantly lower incidence of Covid-19 cases. https://c19p.org/hellwig
62. C. Chaccour, A. Casellas, A. Blanco-Di Matteo, I. Pineda, A. Fernandez-Montero, P. Ruiz-Castillo, M. Richardson, M. Rodríguez-Mateos, C. Jordán-Iborra, J. Brew, F. Carmona-Torre, M. Giráldez, E. Laso, J. Gabaldón-Figueira, C. Dobaño, G. Moncunill, J. Yuste, J. Del Pozo, N. Rabinovich, V. Schöning, F. Hammann, G. Reina, B. Sadaba, and M. Fernández-Alonso, The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial Dec 2020, EClinicalMedicine, Volume 32, Page 100720
EARLY TREATMENT 24 patient ivermectin early treatment RCT: 96% improved symptoms (p=0.05), 95% improved viral load (p=0.01), and 8% improved viral clearance (p=1).
Tiny RCT for early treatment of mild Covid-19 in low-risk patients, with 12 400mcg/kg single-dose ivermectin patients and 12 control patients, showing significantly faster viral load reduction and symptom improvement with ivermectin. https://c19p.org/chaccour
63. S. Abd‐Elsalam, R. Noor, R. Badawi, M. Khalaf, E. Esmail, S. Soliman, M. Abd El Ghafar, M. Elbahnasawy, E. Moustafa, S. Hassany, M. Medhat, H. Ramadan, M. Eldeen, M. Alboraie, A. Cordie, and G. Esmat, Clinical Study Evaluating the Efficacy of Ivermectin in COVID-19 Treatment: A Randomized Controlled Study Jun 2021, J. Medical Virology, Volume 93, Issue 10, Page 5833-5838
LATE TREATMENT 164 patient ivermectin late treatment RCT: 20% shorter hospitalization (p=0.09).
RCT 164 hospitalized patients in Egypt showing lower mortality and shorter hospitalization, but without statistical significance. There were no serious adverse effects. Authors suggest the low dosage may have resulted in lower efficacy than other trials and recommend increased dosage in future trials. Time from symptom onset is not specified. The trial was retrospectively registered and the recruitment start date in the trial registration (June 2020) differs from the paper (March 2020). For other concerns see [onlinelibrary.wiley.com]. https://c19p.org/abdelsalam3
64. E. López-Medina, P. López, I. Hurtado, D. Dávalos, O. Ramirez, E. Martínez, J. Díazgranados, J. Oñate, H. Chavarriaga, S. Herrera, B. Parra, G. Libreros, R. Jaramillo, A. Avendaño, D. Toro, M. Torres, M. Lesmes, C. Rios, and I. Caicedo, Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial Mar 2021, JAMA, Volume 325, Issue 14, Page 1426
EARLY TREATMENT 398 patient ivermectin early treatment RCT: 61% lower progression (p=0.11) and 15% improved recovery (p=0.53).
Phone survey based RCT with low-risk patients, 200 ivermectin and 198 control, showing lower mortality, lower disease progression, lower treatment escalation, and faster resolution of symptoms with treatment, without reaching statistical significance. Authors find the results of this trial alone do not support the use of ivermectin. However the effects are all positive, especially for serious outcomes which are unable to reach statistical significance with the very small number of events in the low-risk population. An open letter, signed by >100 physicians, concluding this study is fatally flawed can be found at jamaletter.com. With the low-risk patient population, there is little room for improvement with an effective treatment– 59/57% (IVM/control) recovered within the first 2 days to either “no symptoms” or “not hospitalized and no limitation of activities;” 73/69% within 5 days. Less than 3% of all patients ever deteriorated. The primary outcome was changed mid-trial. https://c19p.org/lopezmedina
65. M. Munir, A. Khan, and T. Khan, Clinical Disease Characteristics and Treatment Trajectories Associated with Mortality among COVID-19 Patients in Punjab, Pakistan Apr 2023, Healthcare, Volume 11, Issue 8, Page 1192
LATE TREATMENT 1,000 patients ivermectin late treatment study: 48% lower mortality (p=0.13).
Retrospective 1,000 hospitalized Covid-19 patients in Pakistan, showing lower mortality with ivermectin without statistical significance. https://c19p.org/munir
66. A. Zeeshan Khan Chachar, K. Ahmad Khan, M. Asif, K. Tanveer, A. Khaqan, and R. Basri, Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients Sep 2020, Int. J. Sciences-35, Volume 9, Issue 09, Page 31-35
LATE TREATMENT 50 patient ivermectin late treatment RCT: 10% improved recovery (p=0.5).
Small RCT with 25 ivermectin and 25 control patients, not finding a significant difference in recovery at day 7. https://c19p.org/chachar
67. C. Podder, N. Chowdhury, M. Sina, and W. Haque, Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study Sep 2020, IMC J. Med. Science, Volume 14, Issue 2, Page 11-18
LATE TREATMENT 62 patient ivermectin late treatment RCT: 16% faster recovery (p=0.34).
Small RCT with 32 ivermectin patients and 30 control patients. The mean recovery time after enrolment in the intervention arm was 5.31 ± 2.48 days vs. 6.33 ± 4.23 days in the control arm, p > 0.05. Negative PCR results were not significantly different between control and intervention arms, p>0.05. Unclear what the results were because the abstract and Table 5 have switched the results. https://c19p.org/podder
68. H. Tanioka, S. Tanioka, and K. Kaga, Why COVID-19 is not so spread in Africa: How does Ivermectin affect it? Mar 2021, medRxiv
Ivermectin prophylaxis study: 88% lower mortality (p=0.002).
Retrospective study of the 31 onchocerciasis-endemic countries using the community-directed treatment with ivermectin and the 22 non-endemic countries in Africa, showing significantly lower mortality per capita in the countries using ivermectin. https://c19p.org/tanioka
69. D. Camprubí, A. Almuedo-Riera, H. Martí-Soler, A. Soriano, J. Hurtado, C. Subirà, B. Grau-Pujol, A. Krolewiecki, and J. Muñoz, Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients Nov 2020, PLoS ONE, Volume 15, Issue 11, Page e0242184
LATE TREATMENT 26 patient ivermectin late treatment study: 40% lower ventilation (p=0.67), 33% lower ICU admission (p=1), 33% worse improvement (p=1), and 25% worse viral clearance (p=1).
Tiny 26 patients retrospective study of very late treatment with ivermectin 200 μg/kg, median 12 days after symptoms, not showing significant differences. Authors suggest the dose is too low and recommend evaluation of higher doses. All patients received hydroxychloroquine which may reduce the potential benefit for adding ivermectin. https://c19p.org/camprubi
70. F. Gorial, S. Mashhadani, H. Sayaly, B. Dakhil, M. AlMashhadani, A. Aljabory, H. Abbas, M. Ghanim, and J. Rasheed, Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial) Jul 2020, medRxiv
LATE TREATMENT 87 patient ivermectin late treatment study: 42% shorter hospitalization (p<0.0001).
Small trial of hospitalized patients with 16 of 87 patients being treated with ivermectin, showing a significantly lower mean hospital stay with ivermectin: 7.62 vs. 13.22 days, p=0.00005. Zero of 16 ivermectin patients died vs. 2 of 71 control patients. https://c19p.org/gorial
71. H. Pott-Junior, M. Paoliello, A. Miguel, A. Da Cunha, C. De Melo Freire, F. Neves, L. Da Silva de Av´o, M. Roscani, S. Dos Santos, and S. Chach´a, Use of ivermectin in the treatment of Covid-19: a pilot trial Mar 2021, Toxicology Reports, Volume 8, Page 505-510
LATE TREATMENT 31 patient ivermectin late treatment RCT: 85% lower ventilation (p=0.25), 85% lower ICU admission (p=0.25), and 1% improved viral clearance (p=1).
Very small RCT with 4 control patients and 28 ivermectin patients split across 3 different dosage levels, showing lower (non-statistically significant) ICU admission with treatment. Authors suggest that ivermectin for SARS-CoV-2 is safe and reduces symptoms and viral load, and that the antiviral effect appears to be dose-dependent. Retraction/censorship: this paper appears to have been censored at the request of the journal’s founding editor. An external review is mentioned but is not provided, and there is no reply from the authors to C19 group, or indication that the authors were notified. Conclusions in this study are limited due to the small size; however, it was still considered in the context of the full body of research. https://c19p.org/pottjunior
72. F. Cadegiani, A. Goren, C. Wambier, and J. McCoy, Early COVID-19 Therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in Outpatient Settings Significantly Improved COVID-19 outcomes compared to Known outcomes in untreated patients Nov 2020, New Microbes and New Infections, Volume 43, Page 100915
EARLY TREATMENT 24 patients ivermectin early treatment study: 94% lower ventilation (p=0.005) and 98% lower hospitalization (p<0.0001).
Comparison of hydroxychloroquine, nitazoxanide, and ivermectin showing similar effectiveness for overall clinical outcomes in Covid-19 when used before seven days of symptoms, and overwhelmingly superior compared to the untreated Covid-19 population, even for those outcomes not influenced by placebo effect, at least when combined with azithromycin, and vitamin C, D and zinc in the majority of the cases. 585 patients with mean treatment delay 2.9 days. There was no hospitalization, mechanical ventilation, or mortality with treatment.Control group 1 was a retrospectively obtained group of untreated patients of the same population. https://c19p.org/cadegianii
73. S. Hazan, S. Dave, A. Gunaratne, S. Dolai, R. Clancy, P. McCullough, and T. Borody, Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients Jul 2021, Future Microbiology, Volume 17, Issue 5, Page 339-350
LATE TREATMENT 24 patient ivermectin late treatment study: 86% lower mortality (p=0.04) and 93% lower hospitalization (p=0.001).
Small study of 24 consecutive patients in serious condition (9 days post symptoms, mean SpO2 87.4) using combined treatment with ivermectin, doxycycline, zinc, vitamin D, and vitamin C, showing no mortality or hospitalization with treatment. Two patients declined treatment and both died. This study uses a synthetic control arm. https://c19p.org/hazan
74. J. Beltran Gonzalez, M. González Gámez, E. Mendoza Enciso, R. Esparza Maldonado, D. Hernández Palacios, S. Dueñas Campos, I. Robles, M. Macías Guzmán, A. García Díaz, C. Gutiérrez Peña, L. Martinez Medina, V. Monroy Colin, and J. Arreola Guerra, Efficacy and Safety of Ivermectin and Hydroxychloroquine in Patients with Severe COVID-19: A Randomized Controlled Trial Feb 2021, Infectious Disease Reports, Volume 14, Issue 2, Page 160-168
LATE TREATMENT 73 patient ivermectin late treatment RCT: 14% lower mortality (p=1), 9% lower progression (p=1), 37% lower hospital discharge (p=0.71), and 20% longer hospitalization (p=0.43).
RCT late stage severe condition, high-comorbidity hospitalized patients in Mexico with 36 low-dose ivermectin and 37 control patients not finding significant differences. Questions have been raised about this study and the early termination of the study and discontinuation of treatments, because the hospital statistics show a dramatically lower (~75%) case fatality rate during the period of the study: See https://c19p.org/beltrangonzalez
75. Z. Mustafa, C. Kow, M. Salman, M. Kanwal, M. Riaz, S. Parveen, and S. Hasan, Pattern of medication utilization in hospitalized patients with COVID-19 in three District Headquarters Hospitals in the Punjab province of PakistanDec 2021, Exploratory Research in Clinical and Social Pharmacy, Page 100101
LATE TREATMENT 444 patient ivermectin late treatment study: 64% lower mortality (p=0.09).
Retrospective 444 hospitalized patients in Pakistan, showing lower mortality with ivermectin treatment in unadjusted results, not reaching statistical significance. Ivermectin was mostly used with patients in severe, late-stage conditions. Dose ranged from 12mg to 36mg for up to seven days. https://c19p.org/mustafa
76. C. Héctor, H. Roberto, A. Psaltis, and C. Veronica, Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel Nov 2020, J. Biomedical Research and Clinical Investigation, Volume 2, Issue 1
1,195 patient ivermectin prophylaxis study: 100% fewer cases (p<0.0001).
Prophylaxis study using ivermectin and iota-carrageenan showing 0 of 788 cases from treated healthcare workers, compared to 237 of 407 control. See here for discussion of issues with this trial. https://c19p.org/carvalloprep
77. G. Reis, E. Silva, D. Silva, L. Thabane, A. Milagres, T. Ferreira, C. Dos Santos, V. Campos, A. Nogueira, A. De Almeida, E. Callegari, A. Neto, L. Savassi, M. Simplicio, L. Ribeiro, R. Oliveira, O. Harari, J. Forrest, H. Ruton, S. Sprague, P. McKay, C. Guo, K. Rowland-Yeo, G. Guyatt, D. Boulware, C. Rayner, and E. Mills, Effect of Early Treatment with Ivermectin among Patients with Covid-19 Aug 2021, New England J. Medicine
EARLY TREATMENT 1,358 patient ivermectin early treatment RCT: while presented as negative, the co-principal investigator privately reported in an email on April 3, 2022 that “there is a clear signal that IVM works in COVID patients.” Together Trial Ivermectin: impossible data, critical issues, blinding broken, randomization failure, data pledge violation, protocol violations. The trial changed from including vaccinated patients to excluding them on Mar 21, 2021. Disclosed conflicts of interest include Pfizer. One author claimed that a report of ivermectin working as “disinformation.” Also see: 10 Questions for the TOGETHER Trial Investigators, and FDA Reveals Concerns About the Conduct of the TOGETHER Trial, Joining Other Regulators. More at: https://c19p.org/togetherivm
78. T. Ahsan, B. Rani, R. Siddiqui, G. D‘Souza, R. Memon, I. Lutfi, O. I. Hasan, R. Javed, F. Khan, and M. Hassan, Clinical Variants, Characteristics, and Outcomes Among COVID-19 Patients: A Case Series Analysis at a Tertiary Care Hospital in Karachi, Pakistan Apr 2021, Cureus
LATE TREATMENT 165 patient ivermectin late treatment study: 50% lower mortality (p=0.03).
Retrospective 165 already hospitalized (late treatment) patients in Pakistan showing unadjusted lower mortality with combined ivermectin and doxycycline treatment. Details of the ivermectin group compared to other patients are not provided; however ivermectin was given to a similar percentage of patients in the mild, moderate, and severe/critical groups (34.5%, 29.1%, and 36.4%), suggesting that ivermectin treatment was not based on severity. https://c19p.org/ahsan
79. H. Carvallo, Usefulness of Topic Ivermectin and Carrageenan to Prevent Contagion of Covid 19 (IVERCAR) Oct 2020, NCT04425850
229 patient ivermectin prophylaxis study: 96% fewer cases (p<0.0001).
Prophylaxis study using ivermectin and carrageenan showing 0 of 131 cases from treated healthcare workers, compared to 11 of 98 control. The effect is likely to be primarily due to ivermectin – the author has later reported that carrageenan is not necessary. https://c19p.org/carvalloprep2
80. H. Carvallo, H. Roberto, Safety and Efficacy of the Combined Use of Ivermectin, Dexamethasone, Enoxaparin and Aspirin against COVID-19 the I.D.E.A. Protocol Sep 2020, J. Clinical Trials
EARLY TREATMENT 46 patients ivermectin early treatment study: 85% lower mortality (p=0.08).
Prospective trial of ivermectin, dexamethasone, enoxaparin, and aspirin, showing no hospitalization for mild cases, and lower mortality for moderate/severe patients. https://c19p.org/carvallo
81. S. Bhatnagar, A. Elavarasi, H. Raju Sagiraju, R. Garg, B. Ratre, P. Sirohiya, N. Gupta, R. Garg, A. Pandit, S. Vig, R. Singh, B. Kumar, V. Meena, N. Wig, S. Mittal, S. Pahuja, K. Madan, R. Guleria, A. Mohan, T. Dwivedi, R. Gupta, A. Vidyarthi, R. Chaudhry, A. Das, L. Wundavalli, A. Singh, S. Singh, S. Kumar, M. Pandey, A. Mishra, and K. Matharoo, Clinical features, demography, and predictors of outcomes of SARS-CoV-2 infection at a tertiary care hospital in India: A cohort study Aug 2021, Lung India, Volume 39, Issue 1, Page 16
LATE TREATMENT 1,758 patient ivermectin late treatment study: 20% lower mortality (p=0.12).
Retrospective 2,017 hospitalized patients in India, showing lower mortality with ivermectin treatment in unadjusted results. No group details are provided and this result is subject to confounding by indication.https://c19p.org/elavarasi
82. P. Soto-Becerra, C. Culquichicón, Y. Hurtado-Roca, and R. Araujo-Castillo, Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in PeruOct 2020, medRxiv
LATE TREATMENT 2,833 patient ivermectin late treatment study: 17% lower mortality (p=0.01).
Retrospective database study of 5,683 patients, 692 received HCQ/CQ+AZ, 200 received HCQ/CQ, 203 received ivermectin, 1,600 received AZ, 358 received ivermectin+AZ, and 2,630 received standard of care. This study includes anyone with ICD-10 Covid-19 codes which includes asymptomatic PCR+ patients; therefore many patients in the control group are likely asymptomatic with regards to SARS-CoV-2, but in the hospital for another reason. For those that had symptomatic Covid-19, there is also likely significant confounding by indication. In this study all medications show higher mortality at day 30, which is consistent with asymptomatic (for Covid-19) or mild condition patients being more common in the control group. Kaplan Meier curves show that the treatment groups were in more serious condition, and also that after about day 35 survival became better with ivermectin. https://c19p.org/sotobecerrai
83. Ravikirti, A. Ranjan, R. Porel, K. Agarwal, S. Tahaseen, Shyama, and A. Kumar, Association between Ivermectin treatment and mortality in Covid-19: A hospital-based case-control study Apr 2022, Research Square
LATE TREATMENT 965 patient ivermectin late treatment study: 3% lower mortality (p=0.82).
Retrospective 965 late stage (44% severe, 27% ICU) hospitalized patients in India, showing no significant difference with ivermectin treatment. Overall mortality was very high, suggesting very late treatment. The low non-weight-adjusted dose may not be very effective with such late-stage patients. 210 patients were excluded due to early discharge, which may have been patients with earlier onset that are more likely to benefit with ivermectin. Age grouping is very unusual with no breakdown of ages for the 71% of patients >45. Numbers may be unreliable, e.g., cardiovascular disease counts and/or percentages for IVM appear incorrect. Details of adjustments are not provided; there may be extreme confounding by age within the >45 groups which contain the majority of the patients, in addition to confounding by indication. https://c19p.org/ravikirti2
84. S. Roy, S. Samajdar, S. Tripathi, S. Mukherjee, and K. Bhattacharjee, Outcome of Different Therapeutic Interventions in Mild COVID-19 Patients in a Single OPD Clinic of West Bengal: A Retrospective study Mar 2021, medRxiv
EARLY TREATMENT 29 patient ivermectin early treatment study: 6% faster recovery (p=0.87).
Retrospective database analysis of 56 mild Covid-19 patients, all treated with vitamin C, vitamin D, and zinc, comparing ivermectin + doxycycline (n=14), AZ (n=13), hydroxychloroquine (n=14), and standard of care (n=15), finding that all groups recover quickly, and there was no significant difference between the groups. Subject to the usual limitation of a database study, very small size, and limited evaluation of patients. https://c19p.org/roy
85. T. Borody, R. Clancy, Combination Therapy For COVID-19 Based on Ivermectin in an Australian Population Oct 2021, TrialSite News
EARLY TREATMENT 600 patient ivermectin early treatment study: 92% lower mortality (p=0.03) and 93% lower hospitalization (p<0.0001).
Retrospective 600 PCR+ outpatients in Australia treated with ivermectin, zinc, and doxycycline, showing significantly lower mortality and hospitalization with treatment. This trial uses a synthetic control group, and the preliminary report provides minimal details. Notably, advantages include less-biased recruitment (patients do not opt out if they feel they need treatment and don’t want to risk placebo), trials are cheaper, there is less delay in treatment, and trials can be run where it is not ethical to give patients placebo. https://c19p.org/borody
86. J. Vallejos, Ivermectina en agentes de salud e IVERCOR COVID19 Dec 2020, IVERCOR PREP, Preliminary Results
875 patient ivermectin prophylaxis study: 73% fewer cases (p<0.0001).
Report on ivermectin prophylaxis in a hospital in Argentina showing lower cases for healthcare workers taking ivermectin. Results have been published in the press and a presentation posted online; however there is no formal publication to date. These results would be expected to receive priority publication due to the predicted impact on the pandemic and confirmation of previous prophylaxis studies. The lack of formal publication suggests a negative publication bias that may be due to politicization in the authors’ location. Note that this prophylaxis study is different to the Vallejos early treatment trial. https://c19p.org/vallejos
87. S. Szente Fonseca, A. De Queiroz Sousa, A. Wolkoff, M. Moreira, B. Pinto, C. Valente Takeda, E. Rebouças, A. Vasconcellos Abdon, A. Nascimento, and H. Risch, Risk of Hospitalization for Covid-19 Outpatients Treated with Various Drug Regimens in Brazil: Comparative Analysis Oct 2020, Travel Medicine and Infectious Disease, Volume 38, Page 101906
EARLY TREATMENT 717 patient ivermectin early treatment study: 14% higher hospitalization (p=0.53).
Retrospective 717 patients in Brazil showing OR 1.17 [0.72-1.90] for ivermectin. This paper focuses on hydroxychloroquine; event counts for ivermectin are not provided. With significant correlation between the variables used, including overlap in the prescription of multiple treatments that show efficacy alone, and limited data for the model size, the model used here may be inaccurate due to multicollinearity https://c19p.org/fonsecai
88. G. Hayward, L. Yu, P. Little, O. Gbinigie, M. Shanyinde, V. Harris, J. Dorward, B. Saville, N. Berry, P. Evans, N. Thomas, M. Patel, D. Richards, O. Hecke, M. Detry, C. Saunders, M. Fitzgerald, J. Robinson, C. Latimer-Bell, J. Allen, E. Ogburn, J. Grabey, S. De Lusignan, F. Hobbs, and C. Butler, Ivermectin for COVID-19 in adults in the community (PRINCIPLE): an open, randomised, controlled, adaptive platform trial of short- and longer-term outcomes Feb 2024, J. Infection, Page 106130
LATE TREATMENT 5,413 patient ivermectin late treatment RCT: 36% lower long Covid and 16% faster recovery despite very late treatment, low-risk patients, and poor administration. Probability of superiority > 0.999.
PRINCIPLE showed 36% lower ongoing persistent Covid-19 specific symptoms, p<0.0001118, and the primary recovery outcome shows superiority of ivermectin with significantly faster recovery and a probability of superiority > 0.999. While authors make an unprecedented claim that the results are not clinically relevant, 2 days faster recovery and 36% lower long Covid are both highly clinically relevant. Faster recovery is associated with lower mortality. Significantly improved recovery and significantly lower risk of long Covid with ivermectin, despite very late treatment, low-risk patients, and poor administration. Improvement data missing from the abstract (details in link below). The p values for sustained recovery, early sustained recovery, alleviation of all symptoms, and sustained alleviation are all < 0.0001. The efficacy seen for ivermectin here is despite the trial being the most clearly designed to fail trial, with major bias in design, operation, analysis, and reporting. https://c19p.org/principleivm
89. S. Zubair, M. Chaudhry, A. Zubairi, T. Shahzad, A. Zahid, I. Khan, J. Khan, and Muhammad Irfan, The effect of ivermectin on non-severe and severe COVID-19 disease and gender-based difference of its effectiveness Jan 2022, Monaldi Archives for Chest Disease
LATE TREATMENT 188 patient ivermectin late treatment study: 9% higher mortality (p=1) and 8% longer hospitalization (p=0.4).
Retrospective 188 hospitalized patients in Pakistan, 90 treated with ivermectin, showing no significant differences with treatment. The ivermectin group had more severe disease (66% vs 58%, with 6x higher risk for severe disease patients, and more male patients (70% vs. 65%). Higher use of remdesivir and steroids in the ivermectin group also suggests that ivermectin was more likely to be given to patients in more severe condition. There were no side effects seen with ivermectin. Authors note that significantly improved ferritin levels were seen with treatment. Authors state that ivermectin patients received 2 12mg doses, 24 hours apart, but later state that the dosage was not standardized. https://c19p.org/zubair
90. N. Kishoria, S. Mathur, V. Parmar, R. Kaur, H. Agarwal, B. Parihar, and S. Verma, Ivermectin as adjuvant to hydroxychloroquine in patients resistant to standard treatment for SARS-CoV-2: results of an open-label randomized clinical study Aug 2020, Paripex – Indian J. Research, Page 1-4
LATE TREATMENT 32 patient ivermectin late treatment RCT: 8% lower hospital discharge (p=1) and 8% worse viral clearance (p=1).
Small RCT of hospitalized patients in India with 19 ivermectin patients and 13 control patients, with all receiving standard of care including hydroxychloroquine, showing no significant differences. The patient population is biased because the study recruited patients that did not respond to standard treatment. Authors do not specify the treatment delay but it is likely relatively late because the patients had already undergone standard treatment. Criteria for discharge are not provided. The time of discharge status is not specified and may not have been an equal time since treatment initiation for all patients. Authors indicate 19 treatment and 16 control patients, but the results only show 13 control patients. Authors do not indicate why the other 3 are missing. Randomization in this small sample resulted in very large differences in the groups, with over twice as many in the ivermectin group with age >40, and the only 2 patients with age >60 both in the ivermectin group. Authors did not adjust for these… https://c19p.org/kishoria
91. A. Soto, D. Quiñones-Laveriano, J. Azañero, R. Chumpitaz, J. Claros, L. Salazar, O. Rosales, L. Nuñez, D. Roca, and A. Alcantara, Mortality and associated risk factors in patients hospitalized due to COVID-19 in a Peruvian reference hospital Mar 2022, PLOS ONE, Volume 17, Issue 3, Page e0264789
LATE TREATMENT 1,418 patient ivermectin late treatment study: 41% higher mortality (p=0.001).
Retrospective 1,418 very late stage (46% mortality) patients in Peru, showing higher mortality with ivermectin. There is strong confounding by indication; for example 48% of patients with baseline SpO2 <70% were treated compared with 22% for SpO2 >95%. The more extreme Cox result compared to the event counts also supports this. There may also be significant confounding by time with standard of care changing substantially over the first few months of the pandemic. Patients may overlap with those in [Soto-Becerra]. The results in the table and text do not match. https://c19p.org/soto
92. R. Ferreira, R. Beranger, P. Sampaio, J. Mansur Filho, and R. Lima, Outcomes associated with Hydroxychloroquine and Ivermectin in hospitalized patients with COVID-19: a single-center experience Nov 2021, Revista da Associação Médica Brasileira, Volume 67, Issue 10, Page 1466-1471
LATE TREATMENT 102 patient ivermectin late treatment study: 54% higher combined mortality/intubation (p=0.37).
Retrospective 230 hospitalized patients in Brazil showing no significant difference with ivermectin treatment. Authors note that the treatments were more likely to be offered to sicker patients. Authors note that they do not know if treatment was started before or after ICU admission and intubation. Baseline total chest CT opacities were higher for ivermectin (20% vs. 15%). 25% of control patients were admitted within 3 days, compared to 5 days for ivermectin. Only 38% of patients in the ivermectin arm were treated within 7 days, compared to 61% for hydroxychloroquine. These are consistent with ivermectin being used for more severe patients. Dosage is unknown. https://c19p.org/ferreira2
93. J. Vallejos, R. Zoni, M. Bangher, S. Villamandos, A. Bobadilla, F. Plano, C. Campias, E. Chaparro Campias, M. Medina, F. Achinelli, H. Guglielmone, J. Ojeda, D. Farizano Salazar, G. Andino, P. Kawerin, S. Dellamea, A. Aquino, V. Flores, C. Martemucci, S. Martinez, J. Segovia, P. Reynoso, N. Sosa, M. Robledo, J. Guarrochena, M. Vernengo, N. Ruiz Diaz, E. Meza, and M. Aguirre, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial Jul 2021, BMC Infectious Diseases, Volume 21, Issue 1
EARLY TREATMENT 501 patient ivermectin early treatment RCT: 33% lower hospitalization (p=0.23) and 5% worse viral clearance (p=0.55).
RCT with 501 relatively low-risk outpatients in Argentina showing hospitalization OR 0.65 [0.32-1.31]. With only 7% hospitalization, this trial is underpowered. The trial primarily includes low-risk patients that recover quickly without treatment, leaving minimal room for improvement with treatment. 74 patients had symptoms for >= 7 days. Among the 7 patients requiring ventilation, authors note that the earlier requirement in the ivermectin group may be due to those patients having higher severity at baseline. However, authors know the answer to this – it is unclear why it is not reported. There were more adverse events in the placebo group than the ivermectin group, suggesting a possible issue with dispensing or non-trial medication usage. 25+% of patients were hospitalized within 2/3 days for the placebo/treatment groups. https://c19p.org/vallejos2
94. M. Rezai, F. Ahangarkani, A. Hill, L. Ellis, M. Mirchandani, A. Davoudi, G. Eslami, F. Roozbeh, F. Babamahmoodi, N. Rouhani, A. Alikhani, N. Najafi, R. Ghasemian, H. Mehravaran, A. Hajialibeig, M. Navaeifar, L. Shahbaznejad, G. Rahimzadeh, M. Saeedi, R. Alizadeh-Navai, M. Moosazadeh, S. Saeedi, S. Razavi-Amoli, S. Rezai, F. Rostami-Maskopaee, F. Hosseinzadeh, F. Movahedi, J. Markowitz, and R. Valadan, Non-effectiveness of Ivermectin on Inpatients and Outpatients With COVID-19; Results of Two Randomized, Double-Blinded, Placebo-Controlled Clinical Trials Jun 2022, Frontiers in Medicine, Volume 9
EARLY TREATMENT 549 patient ivermectin early treatment RCT: 9% higher ICU admission (p=0.95), 36% higher hospitalization (p=0.41), 2% worse recovery (p=0.49), and 23% worse viral clearance (p=0.16).
RCT 549 low-risk outpatients in Iran. Reported outcomes are very different from the pre-specified outcomes. The inpatient trial is listed separately. The pre-specified primary clinical outcome was not reported. The reported components of this outcome are both positive. Pre-specified outcomes (3 not reported) [irct.ir]: – reduction in persistent cough and tachypnea and O2 saturation above 94% – not reported – negative PCR – reported – main complaints recovery time – not reported (only individual symptoms) – hospitalization – reported – time to hospitalization – not reported – mortality – reported – side effects – reported in only one patient (anomalous) A new outcome “relative recovery” is reported but not mentioned in the trial registration. The reported percentages and RR do not match. Authors include a researcher caught on video admitting that conclusions on ivermectin research were influenced by a funder: https://c19p.org/rezai3
95. D. Buonfrate, F. Chesini, D. Martini, M. Roncaglioni, M. Fernandez, M. Alvisi, I. De Simone, E. Rulli, A. Nobili, G. Casalini, S. Antinori, M. Gobbi, C. Campoli, M. Deiana, E. Pomari, G. Lunardi, R. Tessari, and Z. Bisoffi, High dose ivermectin for the early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof of concept trial Sep 2021, Int. J. Antimicrobial Agents, Page 106516
EARLY TREATMENT 61 patient ivermectin early treatment RCT: 20% improved viral clearance (p=0.59).
Early terminated 89 patient RCT with 29 high dose and 32 very high dose ivermectinpatients, showing dose dependent viral load reduction, although not reaching statistical significance due to early termination. Since most patients have low viral load at day 7, there is little room for improvement with a treatment at day 7. Intermediate results may show significantly greater improvement, but are not provided. Authors note that ivermectin remained safe even at the very high dose used, although tolerability was reduced. Adherence was very low in the very high dose arm (~60%). The paper reports 4 SAEs, all resolved, with 3 patients hospitalized in the very high-dose ivermectin arm, 1 in the high-dose arm, and 0 in the control arm. However, the supplementary data is contradictory, showing 2 grade 3 events in both ivermectin arms (2 infections and infestations, and 2 Covid-19 pneumonia). While this result is not statistically significant, it may be in part due to randomization failure. https://c19p.org/buonfrate
96. L. Shahbaznejad, A. Davoudi, G. Eslami, J. Markowitz, M. Navaeifar, F. Hosseinzadeh, F. Movahedi, and M. Rezai, Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-blind, Randomized, Controlled Clinical Trial Jan 2021, Clinical Therapeutics, Volume 43, Issue 6, Page 1007-1019
LATE TREATMENT 69 patient ivermectin late treatment RCT: 32% faster recovery (p=0.05) and 15% shorter hospitalization (p=0.02).
RCT in Iran showing shorter time to recovery and shorter hospitalization time with ivermectin. There were no adverse effects. There was one death in the treatment group; the patient was in critical condition at baseline and died within 24 hours of admission. Also see [sciencedirect.com] and the author’s response [clinicaltherapeutics.com]. https://c19p.org/shahbaznejad
97. L. Jamir, M. Tripathi, S. Shankar, R. Kakkar, R. Ayyanar, and R. Aravindakshan, Determinants of Outcome Among Critically Ill Police Personnel With COVID-19: A Retrospective Observational Study From Andhra Pradesh, India Dec 2021, Cureus
LATE TREATMENT 266 patient ivermectin ICU study: 53% higher mortality (p=0.13).
Retrospective 266 Covid-19 ICU patients in India, showing significantly lower mortality with PVP-I oral gargling and topical nasal use, and non-statistically significant higher mortality with ivermectin and lower mortality with remdesivir. https://c19p.org/jamir
98. H. Mikamo, S. Takahashi, Y. Yamagishi, A. Hirakawa, T. Harada, H. Nagashima, C. Noguchi, K. Masuko, H. Maekawa, T. Kashii, H. Ohbayashi, S. Hosokawa, K. Maejima, M. Yamato, W. Manosuthi, S. Paiboonpol, H. Suganami, R. Tanigawa, and H. Kawamura, Efficacy and safety of ivermectin in patients with mild COVID-19 in Japan and ThailandSep 2022, J. Infection and Chemotherapy
EARLY TREATMENT 1,029 patient ivermectin early treatment RCT: 205% higher progression (p=0.49), 4% worse improvement (p=0.62), and 4% improved recovery (p=0.72).
RCT very low-risk patients (mean age 35.7, SpO2 97.4) showing no significant differences with rapid recovery and almost no progression in both groups. The groups were unbalanced. There were 41% more patients with dyspnea at baseline in the treatment group. Similarly, at baseline patients with 4+ symptoms scored 2+ were more common in the treatment group – 7% for ivermectin vs. 4% for placebo. Table S8 shows only one case of Covid-19 pneumonia. Authors report 3 and 1 cases of progression; this matches the 3 and 1 cases of the adverse event “Covid-19” in Table S8. It’s unclear how the Covid-19 adverse events were defined since all patients are meant to have Covid-19. Authors’ definition of progression includes “use of Covid-19 therapeutic agents” and therefore the significance for progression of disease is not clear. The study is designed to produce a null result with very low-risk patients, and administered ivermectin on an empty stomach. https://c19p.org/mikamo
99. C. De la Rocha, M. Cid-López, B. Venegas-López, S. Gómez-Méndez, A. Sánchez-Ortiz, A. Pérez-Ríos, R. Llamas-Velázquez, A. Meza-Acuña, B. Vargas-Íñiguez, D. Rosales-Galván, A. Tavares-Váldez, N. Luna-Gudiño, C. Hernández-Puente, J. Milenkovic, C. Iglesias-Palomares, M. Méndez-del Villar, G. Gutiérrez-Dieck, C. Valderrábano-Roldán, J. Mercado-Cerda, J. Robles-Bojórquez, and A. Mercado-Sesma, Ivermectin compared with placebo in the clinical course in Mexican patients with asymptomatic and mild COVID-19: a randomized clinical trial May 2022, BMC Infectious Diseases, Volume 22, Issue 1
EARLY TREATMENT 56 patient ivermectin early treatment RCT: 15% worse recovery (p=0.58) and 2% improved viral clearance (p=0.64).
Small low-risk patient RCT with 30 low-dose ivermectin and 26 control patients, with no primary outcome events in either arm. Viral load was significantly better with ivermectin on day 5, while there was no significant difference on day 1 or day 14. There was no significant difference in combined symptoms; however, authors include cough which was the most frequent symptom and may persist long after infection has been cleared. Ivermectin patients were 4 years older with a higher standard deviation, had higher prevalence of obesity, diabetes, hypertension, and cardiovascular disease, and lower prevalence of hepatic and kidney disease. The slow viral clearance seen may be in part due to acetaminophen use. Authors conclude that “ivermectin is not effective to prevent progression to a severe state;” however there was no progression too severe in either group. https://c19p.org/delarocha
100. C. Bramante, J. Huling, C. Tignanelli, J. Buse, D. Liebovitz, J. Nicklas, K. Cohen, M. Puskarich, H. Belani, J. Proper, L. Siegel, N. Klatt, D. Odde, D. Luke, B. Anderson, A. Karger, N. Ingraham, K. Hartman, V. Rao, A. Hagen, B. Patel, S. Fenno, N. Avula, N. Reddy, S. Erickson, S. Lindberg, R. Fricton, S. Lee, A. Zaman, H. Saveraid, W. Tordsen, M. Pullen, M. Biros, N. Sherwood, J. Thompson, D. Boulware, and T. Murray, Randomized Trial of Metformin, Ivermectin, and Fluvoxamine for Covid-19 Aug 2022, NEJM, Volume 387, Issue 7, Page 599-610
804 patient ivermectin early treatment RCT: 61% lower hospitalization for ivermectin vs. placebo (not reported in the paper which uses a control group including metformin), despite very late treatment, low-risk patients, and poor administration. ER results do not match symptoms.
Covid-OUT remote RCT, showing no significant differences compared to a combined metformin/placebo “control” group. Results for other treatments are listed separately – metformin, fluvoxamine. Authors include metformin patients in the control group, allowing details of adjustments to affect results. Using standard treatment vs. placebo analysis shows 61% lower hospitalization, or 75% lower for patients with onset ≤5 days (not statistically significant with only 7 and 5 events). These results are not reported in the paper or the supplementary appendix, readers need to request the data. There are many major issues with this study. Severity mismatch for ivermectin treatment but not for any other medication or control. Major event counts differ between paper and registry. Baseline data differs between paper and registry. Control group includes metformin, adjustment protocol violation. Primary outcome changes. Multiple outcomes missing, including time to recovery. Protocol page 12 states that “The research team statisticians will remain blinded” while the supplementary data page 40 states that “There is one unblinded statistician with two unblinded supporting statisticians on the study team.” Medication delivery varied significantly over the trial. In this presentation, authors indicate that delivery was initially local, later via FedEx, was much slower in August, there were delays due to team bandwidth issues, and they only realized they could use FedEx same day delivery in September. Treatment was 14 days for metformin and fluvoxamine, but only 3 days for ivermectin. Adherence was very low, with 77% overall reporting 70+% adherence, and 85% for ivermectin reporting 70+% adherence. An author has claimed 85% took all doses but that is contradicted by the 20% reported “Total Interruption or Discontinuation” in Table S2. Authors indicate up to 5-day delay in real-world usage. Authors note up to 11 days treatment delay with a remote clinical trial compared to up to 5 days for “real-world use” @43:00, where the 5 days derive from testing and medical system delays. However, logical real-world use, as used in many locations, is to have the treatment on hand to take immediately upon symptom onset. Control group includes metformin, an adjustment protocol violation. Author claims results from 642 researchers should be censored for false information. Administration on an empty stomach. Results delayed 6 months (including life-saving metformin results) Authors note that “hospitalization is perhaps the most accurate and well-documented end point.” A more detailed analysis of this study due to widespread incorrect press is at: https://c19p.org/covidoutivm
101. A. Krolewiecki, A. Lifschitz, M. Moragas, M. Travacio, R. Valentini, D. Alonso, R. Solari, M. Tinelli, R. Cimino, L. Álvarez, P. Fleitas, L. Ceballos, M. Golemba, F. Fernández, D. Fernández de Oliveira, G. Astudillo, I. Baeck, J. Farina, G. Cardama, A. Mangano, E. Spitzer, S. Gold, and C. Lanusse, Antiviral effect of high-dose ivermectin in adults with COVID-19: A proof-of-concept randomized trial Jun 2021, EClinicalMedicine, Volume 37, Page 100959
EARLY TREATMENT 41 patient ivermectin early treatment RCT: 66% improved viral load (p=0.09).
Proof of concept RCT with 30 ivermectin patients and 15 control patients, showing a concentration dependent antiviral activity, but no significant difference in clinical outcomes. There was no significant difference in viral load reduction between groups overall, but a significant difference was found in patients with higher median plasma ivermectin levels (72% vs. 42%, p=0.004). Mean ivermectin plasma concentration levels correlated with viral decay rate (r=0.47, p=0.02). The change in viral load is provided for the <160ng/mL and >160ng/mL groups, but not the overall treatment group. The corrigendum provides individual viral decay rates for computing the overall treatment group viral decay rate. Authors published a corrigendum. https://c19p.org/krolewiecki
102. S. Naggie, D. Boulware, C. Lindsell, T. Stewart, N. Gentile, S. Collins, M. McCarthy, D. Jayaweera, M. Castro, M. Sulkowski, K. McTigue, F. Thicklin, G. Felker, A. Ginde, C. Bramante, A. Slandzicki, A. Gabriel, N. Shah, L. Lenert, S. Dunsmore, S. Adam, A. DeLong, G. Hanna, A. Remaly, R. Wilder, S. Wilson, E. Shenkman, A. Hernandez, W. Vincent, R. Vincent, R. Bianchi, J. Premas, D. Cordero-Loperena, E. Rivera, M. Gupta, G. Karawan, C. Ziomek, J. Arena, S. DeAlmeida, S. Ramin, J. Nataraj, M. Paasche-Orlow, L. Henault, K. Waite, D. Miller, G. Brounce, C. George-Adebayo, A. Adebayo, J. Wallan, A. Slandzicki et al., Effect of Ivermectin vs Placebo on Time to Sustained Recovery in Outpatients With Mild to Moderate COVID-19: A Randomized Clinical Trial Jun 2022, JAMA, Volume 328, Issue 16, Page 1595
LATE TREATMENT 1,591 patient ivermectin late treatment RCT: 99%, 98%, 97% posterior probability of efficacy for mean time unwell and clinical progression @14 and 7 days, despite very late treatment, low-risk patients, and poor administration. All exceed the pre-specified threshold for superiority. Clinical progression results were changed without explanation in a later version.
Extreme conflict of interest, data inconsistencies, uncorrected errors, no response from authors, participant fraud, refusal to release data, but one would never know that by reading headlines from the New York Times. RCT low-risk outpatients with very late treatment (median 6 days, 25% ≥8 days) in the US, showing 98% probability of efficacy for clinical progression at day 14, a treatment delay-response relationship, and significant efficacy for patients with severe symptoms at baseline. 1) The drugs were sent to the patients by mail, so that some patients took them 13 or 14 days after symptom onset. 2) The authors never saw the vast majority of the patients, and every step of the process was done remotely. They call this a “distributed” trial. 3) There is no reporting of adherence in the trial, so we don’t even know how many of the patients took how many of the doses they were given. 4)There is no per-protocol analysis, so we don’t know how well the drug did in the patients who actually took all the doses.
And still, the results are actually strongly positive for ivermectin, if you strip back the bias. The authors write that there was “a posterior probability of benefit of 0.91.” This is another way to write that they found 91% probability that ivermectin is superior to taking placebo, in shortening time to recovery. The posterior probability ivermectin is effective was 99%, 98%, 97% for mean time unwell and clinical progression @14 and 7 days. All exceed the pre-specified threshold for superiority. Note that the clinical progression results exceeding the superiority threshold in the preprint changed in the journal version for the 400µg/kg arm, with no explanation for over 500 days). The 600µg/kg arm was reported separately by Naggie. When not specified, comments refer to the 400µg/kg (low-dose) arm. The authors didn’t just change the primary endpoint; the ones registered on clinicaltrials.gov (Hospitalizations, Deaths, Symptoms by day 14) aren’t even reported in the paper. The 4th version of the trial protocolmisreports the original endpoint, showing it as being measured 28 days after enrollment, whereas the 1st version of the protocol—as well as clinicaltrials.gov—report it as being measured 14 days after enrollment. “ACTIV-6 made the TOGETHER trial team seem honest in comparison.”
For critique sources, see also: ACTIV-6 Trial on Ivermectin: NIH Scientists Behaving Badly and The Story Of A Real ACTIV-6 Patient and ACTIV-6 Dosing & Timing: A Fox In The Henhouse. There is a more detailed analysis of this study due to widespread incorrect press at: https://c19p.org/activ6ivm
103. P. Sarojvisut, A. Apisarnthanarak, K. Jantarathaneewat, O. Sathitakorn, T. Pienthong, C. Mingmalairak, D. Warren, and D. Weber, An Open Label Randomized Controlled Trial of Ivermectin Plus Favipiravir-Based Standard of Care versus Favipiravir-Based Standard of Care for Treatment of Moderate COVID-19 in Thailand Dec 2022, Infection & Chemotherapy, Volume 54
LATE TREATMENT 317 patient ivermectin late treatment RCT: 104% higher ICU admission (p=0.62), 104% worse improvement (p=0.62), and 4% faster recovery (p=0.63).
RCT low-risk hospitalized patients in Thailand showed no significant difference with the addition of ivermectin to favipiravir based standard of care. Only the abstract is currently available. The trial was registered retrospectively. The primary outcome was WHO-category ordinal scale improvement of 2 points at days 3, 7, 14, 21, for which only a single unspecified time point (when almost all patients have recovered) is provided in the abstract. The registration indicates that the intervention was only provided “after laboratory result”(?) without explanation. https://c19p.org/sarojvisut
Author
Dr. David Gortler is a pharmacologist, pharmacist, research scientist and a former member of the FDA Senior Executive Leadership Team who served as senior advisor to the FDA Commissioner on matters of: FDA regulatory affairs, drug safety and FDA science policy. He is a former Yale University and Georgetown University didactic professor of pharmacology and biotechnology, with over a decade of academic pedagogy and bench research, as part of his nearly two decades of experience in drug development. He also serves as a scholar at the Ethics and Public Policy Center and a 2023 Brownstone Fellow.
View all posts


No comments:
Post a Comment