Reports Of Autopsies In VAERS And Associated Adverse Events Linked To Cause Of Death
Jessica Rose, PhD, MSc, BSc; Independent Researcher and Data Analyst; ORCID: 0000-0002-9091-4425
— Updated: July 26, 2024 —
This is the manuscript I have been working on for a while now, and I finally got my ass in gear to present a draft to you lovely people here. Please feel free to comment prior to my posting to a preprint server! Comments more than welcome. It gets very depressing toward the end and my discussion is not complete.
Reports Of Autopsies In VAERS And Associated Adverse Events Linked To Cause Of Death
Jessica Rose, PhD, MSc, BSc
Independent Researcher and Data Analyst; ORCID: 0000-0002-9091-4425
Correspondence: jessicarose1974@protonmail.com
Abstract
Background: Since the roll-out of the Pfizer-BioNTech BNT162b2, Moderna mRNA-1273, Janssen Ad26.COV2.S and Novavax COVID-19 injections in the United States, millions of individuals have reported adverse events (AEs) using the Vaccine Adverse Events Reports System (VAERS).
Objectives: To examine reports of death in VAERS associated with autopsy reports in the context of the COVID-19 injectable products and Influenza vaccines for equivalent timeframes.
Methods: VAERS data was used to examine the frequency of reporting of general AES, and specific AEs linked to autopsy reports since the beginning of the COVID-19 mass injection campaign - up to and including July 2024. COVID-19 injectable product AE data collected from 2021-2023, were compared to Influenza vaccine AE data collected from 2018-2020, thus matching timeframes of administration and VAERS reporting of AEs associated with each product. The total number of shots administered per product type was also calculated and used to calculate rates of AEs per million doses. Autopsy reports made in association with the COVID-19 injectable products were also further examined in the context of fetal and child deaths. In addition geographic locations were mapped according to ratios of autopsies to deaths per state in order to visualize which states ordered the most autopsies.
Results: The number of autopsy reports in VAERS domestic data following COVID-19 injection spanning 2021-2023 is 18 times higher than the average of Influenza vaccines for the timeframe spanning 2018-2020. This represents a 1,714% increase in absolute number of reports of autopsy for equal timeframes in the context of four COVID-19 products versus twelve Influenza vaccines. The average age of the reports involving autopsy in the COVID-19 shot context is 53 whereas it is 25 in the Influenza shot context. 69% of all COVID-19 shot autopsy-linked reports were associated with cardiovascular AEs, and more precisely, 11%, 12% and 16% of these reports are associated with myocarditis, cardiac arrest and pulmonary embolism (PE), respectively. Likewise for Influenza autopsy-linked reports with regard to percentage of autopsy reports associated with cardiovascular issues (67% of total), but only with 7% associated with myocarditis; there were no autopsy reports involving cardiac arrest or pulmonary embolism. The rate of reporting of autopsies in the COVID-19 shot context decreased significantly (p = 0.03) by a staggering 77.6% when compared to the Influenza shot context indicating a questionable discrepancy between standard autopsy request rates and rates in the COVID-19 era.
Conclusions: Statistically, there is a significant difference between the rate of autopsy reporting when comparing Influenza vaccines to COVID-19 injectable products with. Despite the fact that there is a 1,714% increase in absolute count of autopsies in VAERS when comparing Influenza vaccine to COVID-19 injectable product reports, there is a 77.6% decrease in the rate of autopsy reporting in the context of deaths. 69% (N=262) of autopsy-linked VAERS reports in the context of COVID-19 injections involve cardiovascular AEs including myocarditis, cardiac arrest and PE and supports the theory that the COVID-19 injectable products are deterministic for myocarditis, cardiac arrest and PE. Noteworthy, is that 67% (N=14) of autopsy-linked VAERS reports in the context of Influenza vaccines involve cardiovascular-related death, but with no reported involvement of cardiac arrest or PE.
Keywords
SARS-CoV-2; COVID-19; myocarditis; VAERS; adverse events (AEs); serious adverse events (SAEs)
Word Count: 7,966
Funding source(s): None related
Conflict of interest: Nothing to disclose. Author had access to data and wrote the manuscript.
Background
An autopsy is generally ordered when the cause of death is not known, if there is a public health concern, foul play is suspected or is associated with infants. [1] Pharmacovigilance databases such as VAERS are designed to detect safety signals in data. [2] If safety signals emerge in the context of a particular marketed product, such as a sudden cluster of reports of sudden death or death following cardiac arrest without known etiology, this would provide reason to perform an autopsy. A recent study by McCullough et al., 2024 revealed that of 325 reports of autopsy published in the literature in the context of COVID-19 injectable product administration, that the injections themselves were deterministic of 240 (73.9%), whereby the primary causes of death included cardiovascular-related injuries including sudden cardiac death (35%), pulmonary embolism (12.5%), myocardial infarction (12%), VITT (7.9%), myocarditis (7.1%), multisystem inflammatory syndrome (4.6%), and cerebral hemorrhage (3.8%). [3]
The human cardiovascular system is the means by which blood is circulated throughout the body in order provide oxygen to cells and tissues, remove waste, and regulate blood pressure for physiological function. [4] Defects in this system, at any level – be it the arterial or venous supply routes, can result in disease states ranging from high blood pressure to myocarditis and are often life-threatening. [5,6]
Myocarditis is inflammation of the myocardium or ‘musculature’ of the heart. [7,8] The myocardium is made up of many cell types however the greatest mass of tissue is accounted for by cardiomyocytes. [9,10] Cardiomyocytes are the principal contractile cells and are supported by specialized conduction and stromal cell types including cardiac pericytes. [11] All of these cell types can be damaged by inflammation, and it has recently been shown that SARS-2 spike protein can disrupt human pericyte function. [12]
Pulmonary Embolism (PE) occurs when a blood clot impairs lung function by blocking an artery. It is commonly associated with shortness of breath, coughing up blood and chest pain and can be deadly if not quickly ameliorated. [13]
Both myocarditis and PE can manifest as chest pain, heart failure, or sudden death. [14,15] Myocarditis is a major risk for cardiac death among the young while PE has highest incidence among individuals 70-80 years old. [16,17] The high-risk age population for myocarditis is from puberty through early 30s, and it is the third leading cause of sudden cardiac death in children and young adults. Four per-million children every year were affected by myocarditis before the pandemic. [19,20] It has been reported that 0.05% of all pediatric hospitalizations are for myocarditis. Most cases of myocarditis are identified in young adults with males affected more often than females. [14,19-22] Multiple vaccines have been associated with myocarditis in the past including influenza and smallpox. [23] The incidence of PE is estimated to be approximately 60 to 70 per 100,000 individuals of any general population. [17]
Rare cases of myocarditis and PE have been reported after SARS-CoV-2 infection before the advent of COVID-19 injections. [24-28] None of these cases resulted in cardiac hospitalization and death, and as a result, myocarditis screening programs for athletes and soldiers were dropped by the end of 2020. In the context of COVID-19 critical illness, multiple studies have reported cardiac injury defined by clusters of ICD codes related to cardiac troponin measurement. [29,30] None of these reports used clinical adjudication in addition to troponin levels or ICD codes with physician review of symptoms, electrocardiogram (ECG/EKG), echocardiography, cardiac magnetic resonance imaging (MRI) and clinical symptoms.
COVID-19 injection-induced myocarditis can be defined as the onset of clinical myocarditis that is temporally associated with COVID-19 mRNA or adenoviral DNA injection administration and in the absence of another known cause. Common clinical symptoms after vaccination include chest pain, palpitations, and effort intolerance. [31-34] Thus, the clinical diagnosis calls for characteristic symptoms of cardiac arrest, elevated troponin levels, ECG changes (diffuse ST segment elevation) and in some cases left and right ventricular dysfunction on echocardiography. In cases where the diagnosis is not secure, cardiac MRI can detect changes in tissue characterization (late gadolinium enhancement) consistent with myocardial inflammation. [33-37]
VAERS was first implemented in 1990 by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) to receive reports about adverse events that may be associated with injections. [2,38] The primary purpose for maintaining the database is to serve as an early warning or signaling system for adverse events not detected during pre-market testing. In addition, the National Childhood Injection Injury Act of 1986 (NCVIA), which was created because injections cause “unavoidable harm,” requires health care providers and injection manufacturers to report to the DHHS specific adverse events following the administration of those injections outlined in the Act [39] Under-reporting is a known and serious disadvantage of the VAERS system. [2,40,41]
An Adverse Event (AE) is defined as any untoward or unfavorable medical occurrence in a human study participant, including any abnormal physical exam or laboratory finding, symptom, or disease, temporally associated with the participants’ involvement in the research, whether or not considered related to participation in the research. A serious or severe adverse event (SAE) is defined as any adverse event that results in death, is life threatening, or places the participant at immediate risk of death from the event as it occurred, requires, or prolongs hospitalization, causes persistent or significant disability or incapacity, results in congenital anomalies or birth defects or is another condition which investigators judge to represent significant hazards. [2,38,42] These classifications are based on the Code of Federal Regulations. The VAERS handbook states that 10-15% of reported AEs are classified as severe for any given set of data. [2] Myocarditis qualifies as an SAE as it can be associated with hospitalization and unpredictable cardiac arrest with sudden death.
Approximately 81% of the United States population has received at least one COVID-19 shot, according to CDC data as of May 10, 2023. [43] The BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen) and Novavax products had not been fully licensed by the U.S. Food and Drug Administration (FDA) prior to August 23, 2021 [44], and were instead authorized for emergency use by the FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19). [45] The injections were not approved to reduce transmission or the severity of infection with SARS-CoV-2. None of the products were shown to reduce the risk of COVID-19 hospitalization or death in prospective, randomized, double-blind, placebo-controlled trials. Thus, any emergent AEs resulting in hospitalization and death associated with these products would tip the risk-benefit ratio toward risk – authorized or not.
The roll-out of COVID-19 injections are actively being monitored by regulatory agencies, but all of the risks are not yet known. [46-49] Recently, the Israeli Ministry of Health announced that approximately 1 in 4,500 men ages 16 to 24 who received BNT162b2 developed myocarditis. [50] In prospective cohort studies with measures before and after the second and third injections, Mansanguan and Buergin reported rates of possible myocarditis of 2.3% and 2.8%, respectively. [51,52] There is great concern regarding a causal link between myocarditis and the COVID-19 injections. [52-57] Thus, we set out to describe the raw data in VAERS and the COVID-19 injections as potential determinants of myocarditis.
Methods
To analyze the VAERS data the Language and Environment for Statistical Computing, R, was used. Excel was also used to generate some of the figures. The VAERS data is available for download in three separate comma separated values (csv) files representing i) general data for each report; ii) the reported AEs or ‘symptoms’, and iii) injection data including injection manufacturer and lot number, for each report. The VAERS dataset is updated once a week and has a one-week lag time between report updates. Upon individual reporting of injection side effects or AEs, a temporary VAERS ID number is assigned to the individual to preserve confidentiality, and a detailed description of the side effects are transcribed along with the individual’s age, residence by state, past medical history, allergies and sex and other details. In addition, the vax lot number, place of injection and manufacturer details are included in the report. If the VAERS report is ‘validated’ following vetting, a permanent VAERS ID is assigned, and the report is filed in the front-end data set available for download.
In order to maximize the input variables per individual for analysis, the three files were merged using the VAERS ID as a linking variable. The merged data sets comprise data collected pertaining to all reported AEs associated with BNT162b2, mRNA-1273, Ad26.COV2.S and Novavax products: the four injection manufacturers responsible for nCoV-2019 products currently being administered in the United States, or pertaining to all reported AEs associated with Influenza vaccines from 2018-2020. Data was filtered according to injection type (COVID19-1 (monovalent) and COVID19-2 (bivalent)) or Influenza products [58], and relevant variables were sorted including VAERS ID, AEs, age, sex, state, vaccination date, date of death, death, injection dose series, injection lot number, injection manufacturer, hospitalization, emergency department visit and onset date of AEs. Autopsy, myocarditis, cardiac arrest and pulmonary embolism as standalone AEs were extracted by keyword, and cardiac events were grouped by extracting multiple keywords according to MedDRA nomenclature. [59]
Vaccination and excess mortality data were also downloaded from the Our World in Data database. [60]
Results
All-Cause Adverse Events
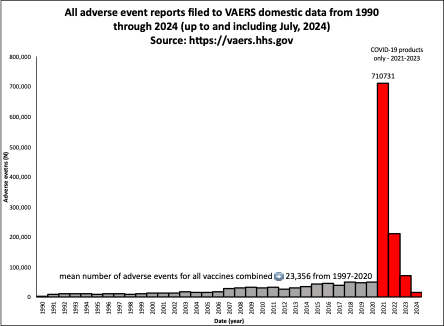
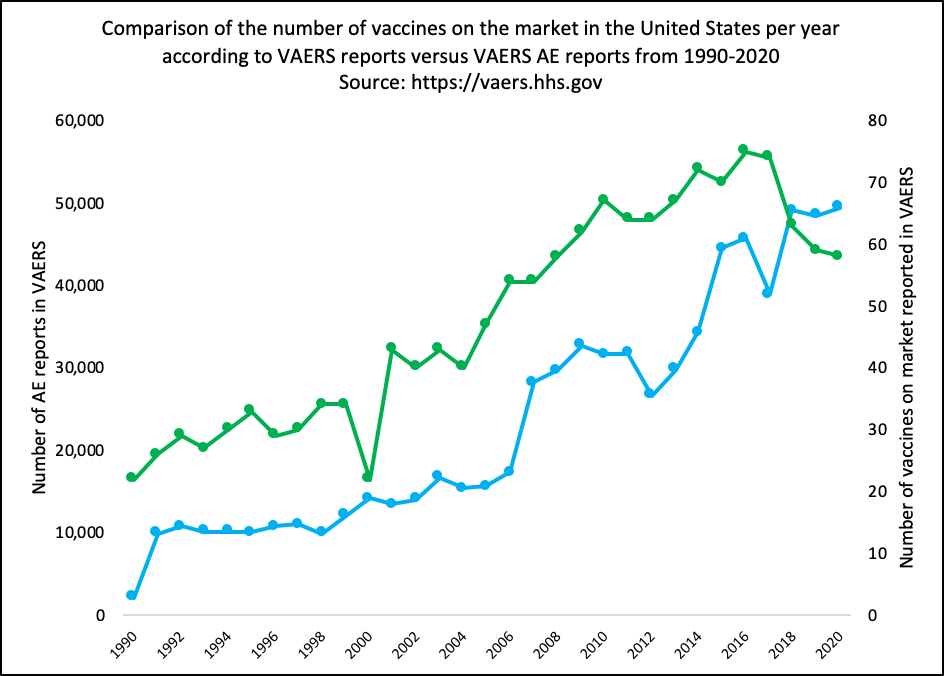
As of July 2024, 1,002,624 domestic reports have been reported to the VAERS system in the context of the COVID-19 injections for public download (https://vaers.hhs.gov). When comparing this number to total number of AE reports filed to VAERS for the past 30 years for all vaccines combined, the number of reports in the context of the COVID-19 injections are disproportionately high (Figure 1). Note that the VAERS reports for 2021 onward are for the COVID-19 injections only. The average number of AE reports per year for all injections combined for the past 30 years is 23,356 and during this time period, the number of reports only slightly increased (Figure 1 – grey bars). The increase in AEs has been proportional to the increase in the number of vaccine products the entering the market prior to COVID-19 injections. (Supplementary Figure 1)
Figure 1: All AEs filed to VAERS domestic data from 1990 through to July 2024. The grey bars represent all vaccines combined and the red bars represent only the COVID-19 injectable products.
In 2021, for the COVID-19 injectable products only, 710,731 reports were filed. Between 2020 and 2021, there was a 1,338% increase in reports. This is not due to the greater number of injections administered as demonstrated by quantitative comparison of the COVID-19 injections and only the influenza injections for a 462-day timeframe: although there were 2.3 times as many COVID-19 products compared with influenza injections administered in this timeframe.
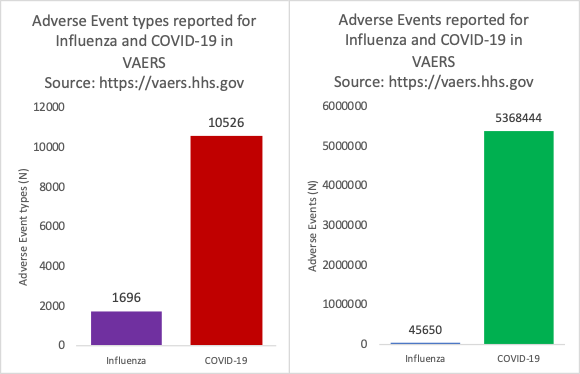
Figure 2: VAERS reports of adverse events by type (left) and absolute count (right) for Influenza vaccines and COVID-19 injectable products spanning a 462-day timeframe.
As shown in Figure 2, there were 6.2 times as many AE types reported by MedDRA code and 118 times as many AE reports. More than 14,000 different AE types by MedDRA code have been reported as of July 2024 following the initial roll-out of the COVID-19 injectable products. The number of types of AEs by MedDRA code reported for all other injections combined in 2020 is only 5000.
Death Adverse Events for COVID-19 injectable products up to an including July 2024
As of July 2024, there are 20,866 reports of death in VAERS: spontaneous abortion (fetal death) inclusive. This comprises 2.1% of total reports. Of these reports, 51.2% are associated with a cardiovascular AE. Of these reports, 387 (1.85%) have autopsies listed as an associated AE and of these reports, 69% are associated with cardiovascular AEs. Interestingly, of the total deaths, 218 involve sudden and/or unexpected death. There are also 34 reports where it is clearly stated that the cause of death is unknown. In other cases, cause of death is clearly stated in the VAERS report such as is the case with VAERS_ID: 2745505: Patient is deceased - cause of death is ischemic cardiomyopathy. This male was 56 years old, on his 6th dose, and died 13 days following this dose.
VAERS reports for Influenza vaccines 2018-2020/COVID-19 injectable products 2021-2023
Descriptive statistics for VAERS data relating to Influenza vaccines (2018-2020) and COVID-19 injectable products (2021-2023) are shown in Table 1.
Table 1: Descriptive statistics for VAERS data relating to Influenza vaccines (2018-2020) and COVID-19 injectable products (2021-2023).
The reporting rate of total AEs per million doses in the context of the COVID-19 shots is 21 times higher than for the Influenza vaccines. More specifically, the percentage of deaths per total AEs is 3 times higher in the COVID-19 shot context. Deaths associated with cardiovascular AEs are 1.7 times higher in the context of the COVID-19 shots with almost half of the deaths co-associated with cardiovascular AEs in the latter context. Perhaps most interesting, however, is the rate of autopsy reporting in the context of death AEs when comparing Influenza vaccine to COVID-19 injectable product reports. Despite the fact that there is a 1,714% increase in absolute count of autopsies in VAERS when comparing Influenza vaccine to COVID-19 injectable product reports, there is a 77.6% decrease in the rate of autopsy reporting in the context of deaths. This is quite a remarkable finding and begs the question: Why weren’t more autopsies ordered in the context of individuals who died within temporal proximity to being administered a COVID-19 injectable product? 18% and 24% of the COVID-19 injectable product-associated deaths reported to VAERS were reported within 3 and 7 days of injection. The necessity and importance of autopsy following injection-associated death has been detailed in a publication by Walach, Klement and Aukema in 2021. [61]
Chi-square tests (results significant at p < 0.05) confirm statistically significant differences between Influenza and COVID-19 shots with respect to whether or not autopsies were performed subsequent to death (X2 (1, N = 1026649) = 4.4659, p = 0.03). Similarly, chi-square tests confirm statistically significant differences when comparing Influenza and COVID-19 shot death counts (X2 (1, N = 1026649) = 299.251, p < 0.00001). Interestingly, there is no statistically-significant difference between the reports of autopsy with cardiovascular involvement when comparing Influenza and COVID-19 shots (X2 (1, N = 1026649) = 2.5945, p < 0.1). Perhaps the autopsies ordered in the Influenza context should be re-examined as well.
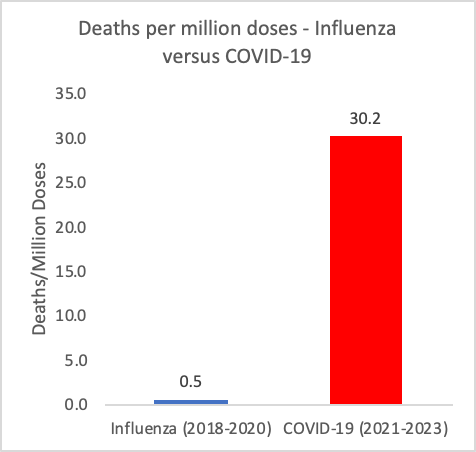
Figure 3: Deaths reported to VAERS per million doses reported by the CDC for Influenza vaccines administered between 2018 and 2020 and COVID-19 injectable products administered between 2021 and 2023. https://vaers.hhs.gov; https://covid.cdc.gov/covid-data-tracker/#vaccination-states-jurisdictions
In addition to comparing the death rates per total AEs, the death rates per million doses can be calculated in each product context for three-year equivalent timeframes. The death rates per million doses are significantly different when comparing the COVID-19 shots to Influenza shots, as shown in Figure 3, whereby in the COVID-19 shot context it is 60 times higher. This means that the conjecture that the anomalously high number of death reports filed to VAERS, and reports to VAERS in general in the context of the COVID-19 injectable products, are due to ‘more shots having been administered’ is demonstrably false. The number of deaths per million doses in the COVID-19 shot context is statistically-significantly higher (X2 (1, N = 862728782) = 5069.5289, p < 0.00001).
Autopsies reported in VAERS in the context of the COVID-19 injectable products
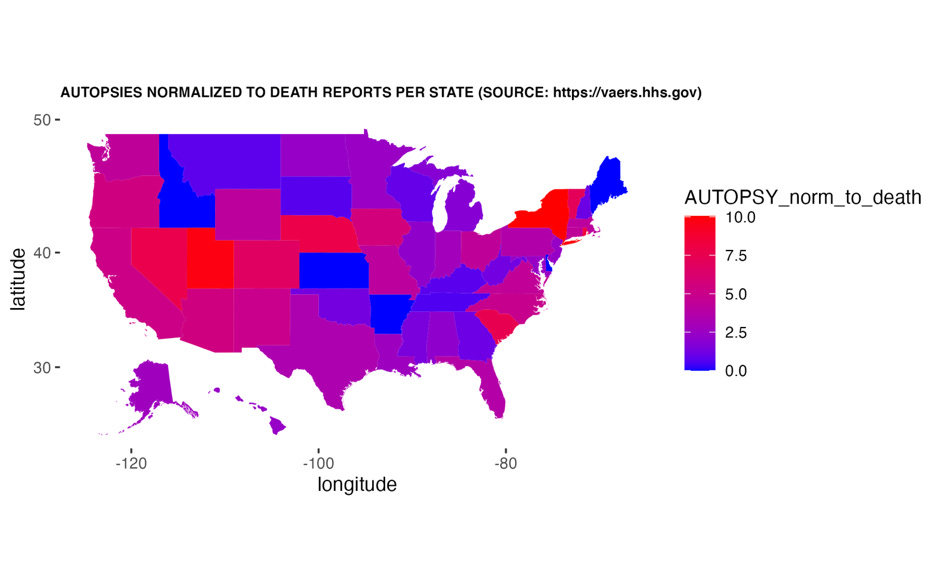
During the timeframe spanning 2021-2023, a total of 381 reports of COVID-19 injection-related autopsies were reported to VAERS. Shockingly, this only represents 1.9% of total death reports filed to VAERS. Of these reports, 69% involved cardiovascular AEs. Because of the nature of the VAERS reporting system, it is possible to determine what percentage of specific types of AEs were associated with reports where an autopsy is also reported as an AE. As previously stated, myocarditis, cardiac arrest and pulmonary embolism are frequently reported AEs in the context of the COVID-19 injectable products and oftentimes in the context of a death report. Of the autopsy reports with associated cardiovascular AEs, 11%, 12% and 16% were concurrent with myocarditis, cardiac arrest and pulmonary embolism, respectively. The percentages of people who had autopsies who died are shown per state in the map in Figure 4. New York and Utah were the top 2 states at 10.1% and 9.8%.
Figure 4: Distribution of autopsies according to percentage performed as per death reports in VAERS per state.
If the number of states requesting autopsies in the context of sudden deaths or fetal deaths was higher, we would know more regarding etiology.
Closer examination of COVID-19 injectable product autopsy reports in miscarriage/still birth context
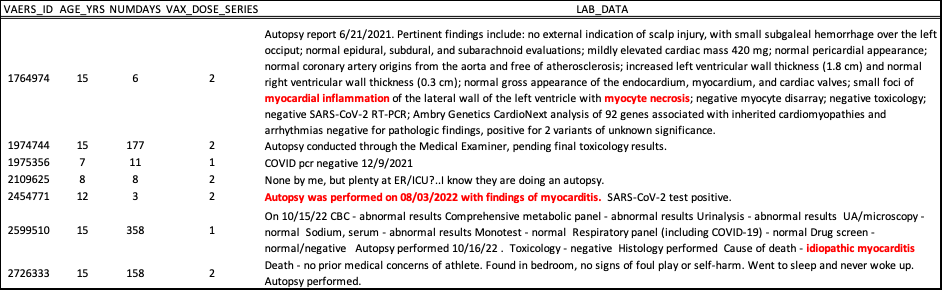
Many pregnant women are still being told by authorities that they should be injected with COVID-19 injectable products despite the fact that SARS-2 has evolved away from any original potential pathogenicity, and that there is no long-term safety data for pregnant women in the context of these products. This recommendation is maintained on the CDC website as of July 2024. [62,63] Reports of “Exposure during pregnancy” or “Maternal exposure during pregnancy” are currently at 5,259 in the VAERS system. Of the 6 autopsy reports in VAERS that involve infants who had autopsies, 2 are pending results as per laboratory data (LAB_DATA). Of the 4 reports with laboratory data, the results of the autopsies were all inconclusive as indicated in Table 2.
Table 2: Reports of fetal deaths associated with the COVID-19 injectable products where autopsies were ordered and reported to VAERS. Shown are the VAERS_IDs (VAERS_ID), the free text (SYMPTOM_TEXT) and laboratory findings (LAB_DATA), if any. https://vaers.hhs.gov
Of the 4 fetal death reports in VAERS, one report (VAERS_ID: 1640972) states that the baby stopped growing on the day of her COVID-19 shot. It was following the Moderna shot and the dose is unknown. The lab reports indicate that the fetus indeed did not increase in weight the week after injection and also that the amniotic fluid index had decreased. This woman had gotten pregnant via in vitro fertilization (IVF) – a very expensive procedure – and was quite far along in her pregnancy (17 weeks). Another new mother (VAERS_ID: 1602762) had her infant die 10 days after birth 45 days after her Moderna shot. The dose number is not known. Presumably, based on her VAX_DATE and ONSET_DATE, she got her shot at 8 months pregnant, gave birth and then 10 days later lost her child. This is the first report of late term injection associated with infant demise I have reported on. In her case, the autopsy results are not ‘back yet’ and it is likely that these results will never see the VAERS database. In any case, the only listed AEs for this woman’s report are “Autopsy” , “Exposure during pregnancy” and “Death of relative”, so with this data, it is impossible to assess true cause of death.
The tragedy here cannot be under-expressed.
Closer examination of COVID-19 injectable product autopsy reports in childhood context
Of the 7 children listed in VAERS with autopsy reports, there are 3 that indicate ‘idiopathic’ myocarditis as the cause of death. Something induced myocarditis in these children and in these specific cases, death occurred 6 (2 doses), 3 (2 doses) and 358 (1 dose) days after their last shot of Pfizer injectable product. In the other 4 cases, there is no data indicative of cause of death.
Table 3: Reports of child (<=15 years of age) deaths associated with the COVID-19 injectable products where autopsies were ordered and reported to VAERS. Shown are the VAERS_IDs (VAERS_ID), ages (AGE_YRS), the number of days that passed between injection and death (NUMDAYS), the dose number (VAX_DOSE_SERIES) and laboratory findings (LAB_DATA), if any. https://vaers.hhs.gov
Discussion
Autopsies are essential to discovery of cause of death. Considering the fact that 18% and 24% of the COVID-19 injectable product-associated deaths reported to VAERS were reported within 3 and 7 days of injection, it is not a stretch to question the etiology as being injection-induced. It is also telling that in a cohort of individuals in this study, that idiopathic myocarditis was listed as the cause of death following autopsy. Autopsies should, in fact, be a requirement considering the evidences of COVID-19 injectable product-induced death etiology. [64-68]
The number of autopsy reports in VAERS domestic data following COVID-19 injection spanning 2021-2023 is 18 times higher than for Influenza vaccines for the timeframe spanning 2018-2020. This represents a 1,714% increase in absolute number of reports of autopsy for equal timeframes in the context of 4 COVID-19 products, versus 12 Influenza vaccines. It is as of yet, unexplained, why there is such a discrepancy in the absolute counts of reports of both deaths and autopsies in the COVID-19 injectable product context. Perhaps more concerning however, is the 77.6% decrease in the rate of autopsy reporting to VAERS when comparing Influenza vaccines to the COVID-19 injectable products. In a time when the importance and relevance of autopsies is so great, one would think that the rate of autopsies ordered in the context of the COVID-19 crisis would be at least as high as for previous vaccine contexts.
The cause of the still births and fetal deaths in the context of the COVID-19 injectable products has not been ascertainable by autopsies ordered, according to VAERS data. The etiology must be sought out by asking questions that perhaps we have not been permitted to ask to date, such as, are the COVID-19 shots the cause of these fetal deaths and if so, how? Until we know that the COVID-19 shots did not cause these fetal deaths, we cannot assume that they did not play a role. Further investigations should be carried out as to etiology, and more autopsies should be ordered.
Emerging sources of clinical and peer-reviewed data supporting the conclusion that COVID-19 injections are deterministic for myocarditis, including fatal cases, are growing. Given the very low SARS-CoV-2 infection fatality rate (IFR) in children with robust natural immune responses [69,70] and the presence of effective medical treatment and prevention [71,75], COVID-19 product injection – especially novel modified mRNA-LNP-based injection - poses more harm to children than theoretical benefit. Considering the plethora of published studies and case studies confirming cardiovascular involvement with death of young people, athletes and others in the context of a temporal association to injection with a COVID-19 product, deaths associated with cardiovascular AEs must be accurately reported and autopsies ordered. [76-81] Because of the spontaneous reporting of events to VAERS, we can assume that the cases reported thus far are not rare, but rather, just the tip of the iceberg. Under-reporting is a known and serious disadvantage of the VAERS system. Thus, VAERS alone without adjustment, cannot be used to estimate population incidence. Based on the 20,425 death reports filed to VAERS as of December 2023, using an under-reporting factor of 31 [41], it is estimated that the actual number of COVID-19 injectable product-associated deaths in the United States is 633,175.
Safety signals emerging from VAERS were apparent in January of 2021. [41] Reports of death after product administration should prompt market withdrawal. Historically, there are many examples of biological product recalls. In 2010, rotavirus injections licensed in the U.S were found to contain Porcine circovirus (PCV) type 1 and were subsequently suspended. [82] In 2010, an increased risk of narcolepsy was found following vaccination with a monovalent H1N1 influenza injection that was used in several European countries during the H1N1 influenza pandemic. [83] Between 2005 and 2008, a meningococcal injection was suspected to cause Guillain Barré Syndrome (GBS). [84] In 1998, an injection designed to prevent rotavirus gastroenteritis was associated with childhood intussusception after being vaccinated. [85-86] Finally, in the early 2000s, a hepatitis B injection product was linked to multiple sclerosis (MS). [87]
Children have a negligible risk for COVID-19 [88], and yet they are a high-risk group for myocarditis from COVID-19 injectable product use. [57] Cardiac abnormalities have been detected for at least a year after the initial diagnosis of COVID-19 injection-induced myocarditis. [89] The exact mechanisms of action for induction and progression of COVID-19 injection-induced myocarditis, and death, need to be elucidated to ensure appropriate management of both AEs and products.
Limitations of this study are acknowledged and are based on use of a pharmacovigilance database where reporting of AEs is not mandatory. VAERS data are grossly under-reported due to many reasons, including the lack of clinical recognition of injury in the context of the COVID-19 injectable products, frustration with the VAERS online system, and fear of professional reprisal. In addition, and as a specific example, despite myocarditis being the MedDRA code listed in VAERS, the diagnosis of myocarditis requires clinical adjudication in order to be deemed correct. Thus myocarditis may be under-reported even more so. Also limiting with regard to etiology, autopsies don’t always reveal the cause of death, and this is more likely to be the case in the COVID-19 era because medical professionals are asking the wrong questions, or rather, not asking the right questions. The very first question a coroner should ask is: “Did the deceased get a COVID-19 shot.” The second question should be: “When did they receive their last COVID-19 shot?” Ascertaining the cause and manner of death, wherever possible, should be a priority. [90]
Conclusion
This study demonstrates the reduction of autopsy requests in the context of a specific product and the question is: Why? The public was convinced that these experimental COVID-19 injectable products were necessary in order to mitigate a health disaster in humans, and experimental COVID-19 injectable products – including ones based on two novel technologies (modified mRNA and lipid nanoparticle) [91,92] were rushed to market based on the supposition that we were in an emergency situation. [93,94] If the public are to believe that there was a need to expedite experimental products to vanquish SARS-2, then the public can also believe that autopsies should also be expedited as well; especially considering the significantly higher rate of death in the context of the COVID-19 injectable products when compared to Influenza vaccines alone. Even though autopsies don’t always reveal the cause of death, without them, we have no definitive answers at all.
Supplementary Material
Supplementary Figure 1: Comparison of the number of vaccines on the market in the United States per year according to VAERS reports versus VAERS AE reports from 1990-2020.
References
1. Odendaal, H. Consent for Autopsy Research for Unexpected Death in Early Life, Obstetrics and Gynecology, January 2011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3268257/
2. Vaers Data Use Guide - HHS.gov [Internet]. Department Of Health And Human Services; 2020 Available from: https://vaers.hhs.gov/docs/VAERSDataUseGuide_November2020.pdf
3. Nicolas Hulscher, Paul E. Alexander, Richard Amerling, Heather Gessling, Roger Hodkinson, William Makis, Harvey A. Risch, Mark Trozzi, Peter A. McCullough, A Systematic REVIEW of Autopsy findings in deaths after covid-19 vaccination, Forensic Science International, 2024, 112115, ISSN 0379-0738, https://doi.org/10.1016/j.forsciint.2024.112115
4. Cooper LT Jr. Myocarditis. N Engl J Med. 2009 Apr 9;360(15):1526-38. doi: 10.1056/NEJMra0800028. PMID: 19357408; PMCID: PMC5814110. https://pubmed.ncbi.nlm.nih.gov/19357408/
5. Oliver, Michael Francis, Entman, Mark L. and Jacob, Stanley W. "human cardiovascular system". Encyclopedia Britannica, 19 Apr. 2024, https://www.britannica.com/science/human-cardiovascular-system
6. Desai AN. High Blood Pressure. JAMA. 2020;324(12):1254–1255. doi:10.1001/jama.2020.11289. https://jamanetwork.com/journals/jama/fullarticle/2770851
7. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012 Feb 25;379(9817):738-47. doi: 10.1016/S0140-6736(11)60648-X. Epub 2011 Dec 18. PMID: 22185868; PMCID: PMC5814111. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60648-X/abstract
8. Camm, A. John and others (eds), The ESC Textbook of Cardiovascular Medicine, 3 edn, The European Society of Cardiology Series (Oxford, 2018; online edn, ESC Publications, 1 July 2018), https://doi.org/10.1093/med/9780198784906.001.0001, accessed 15 Aug. 2023.
9. Libby P, Swirski FK, Nahrendorf M. The Myocardium: More Than Myocytes. J Am Coll Cardiol. 2019 Dec 24;74(25):3136-3138. doi: 10.1016/j.jacc.2019.10.031. PMID: 31856970
10. Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1883-91. doi: 10.1152/ajpheart.00514.2007. Epub 2007 Jun 29. PMID: 17604329
11. Weinhaus A.J., Roberts K.P. (2009) Anatomy of the Human Heart. In: Iaizzo P. (eds) Handbook of Cardiac Anatomy, Physiology, and Devices. Humana Press. https://doi.org/10.1007/978-1-60327372-5_5
12. Avolio E, et al. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signaling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci (Lond). 2021 Dec 22;135(24):2667-2689. doi: 10.1042/CS20210735. PMID: 34807265; PMCID: PMC8674568. https://doi.org/10.1042/CS20210735
13. Essien EO, Rali P, Mathai SC. Pulmonary Embolism. Med Clin North Am. 2019 May;103(3):549-564. doi: 10.1016/j.mcna.2018.12.013. PMID: 30955521. https://doi.org/10.1016/j.mcna.2018.12.013
14. Harris KM, Mackey-Bojack S, Bennett M, Nwaudo D, Duncanson E, Maron BJ. Sudden Unexpected Death Due to Myocarditis in Young People, Including Athletes. Am J Cardiol. 2021 Mar 15;143:131134. doi: 10.1016/j.amjcard.2020.12.028. Epub 2020 Dec 19. PMID: 33347841. https://doi.org/10.1016/j.amjcard.2020.12.028
15. Markwerth P, Bajanowski T, Tzimas I, Dettmeyer R. Sudden cardiac death-update. Int J Legal Med. 2021 Mar;135(2):483-495. doi: 10.1007/s00414-020-02481-z. Epub 2020 Dec 21. PMID: 33349905; PMCID: PMC7751746. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7751746/
16. Singer ME, Taub IB, Kaelber DC. Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis. medRxiv [Preprint]. 2022 Mar 21:2021.07.23.21260998. doi: 10.1101/2021.07.23.21260998. PMID: 34341797; PMCID: PMC8328065. https://pubmed.ncbi.nlm.nih.gov/34341797/
17. Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013 Spring;18(2):129-38. PMID: 23940438; PMCID: PMC3718593. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3718593/
18. Ammirati E, et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ Heart Fail. 2020 Nov;13(11):e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405. Epub 2020 Nov 12. PMID: 33176455; PMCID: PMC7673642. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007405
19. Peretto G, et al. Arrhythmias in myocarditis: State of the art. Heart Rhythm. 2019 May;16(5):793-801. doi: 10.1016/j.hrthm.2018.11.024. Epub 2018 Nov 24. PMID: 30476544. https://doi.org/10.1016/j.hrthm.2018.11.024
20. Kim J, Cho MJ. Acute Myocarditis in Children: a 10-year Nationwide Study (2007-2016) based on the Health Insurance Review and Assessment Service Database in Korea. Korean Circ J. 2020 Nov;50(11):1013-1022. doi: 10.4070/kcj.2020.0108. Epub 2020 Aug 7. PMID: 32812406; PMCID: PMC7596206. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7596206/
21. Arola A, Pikkarainen E, Sipilä JO, Pykäri J, Rautava P, Kytö V. Occurrence and Features of Childhood Myocarditis: A Nationwide Study in Finland. J Am Heart Assoc. 2017 Nov 18;6(11):e005306. doi: 10.1161/JAHA.116.005306. PMID: 29151030; PMCID: PMC5721735. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5721735/
22. Fairweather D, Beetler DJ, Musigk N, Heidecker B, Lyle MA, Cooper LT Jr, Bruno KA. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front Cardiovasc Med. 2023 Mar 2;10:1129348. doi: 10.3389/fcvm.2023.1129348. PMID: 36937911; PMCID: PMC10017519. https://doi.org/10.3389/fcvm.2023.1129348
23. Engler RJ, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015 Mar 20;10(3):e0118283. doi: 10.1371/journal.pone.0118283. PMID: 25793705; PMCID: PMC4368609. https://doi.org/10.1371/journal.pone.0118283
24. Daniels CJ, et al.; Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: Results From the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021 Sep 1;6(9):1078-1087. doi: 10.1001/jamacardio.2021.2065. PMID: 34042947; PMCID: PMC8160916. https://jamanetwork.com/journals/jamacardiology/fullarticle/2780548
25. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463-1471. doi:10.1016/j.hrthm.2020.05.001. https://doi.org/10.1016/j.hrthm.2020.05.001
26. Castiello T, Georgiopoulos G, Finocchiaro G, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges [published online ahead of print, 2021 Mar 24]. Heart Fail Rev. 2021;1-11. doi:10.1007/s10741-021-10087-9. https://link.springer.com/article/10.1007/s10741-021-10087-9
27. Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021 Jan 23:1–7. doi: 10.1007/s11739-021-02635-w. Epub ahead of print. PMID: 33484452; PMCID: PMC7823176. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7823176/
28. Ramphul K, et al. Trends in admissions for COVID-19 in the United States between April 2020 and December 2021 and cardiovascular events. Arch Med Sci Atheroscler Dis. 2024 Mar 30;9:e60-e65. doi: 10.5114/amsad/185410. PMID: 38846059; PMCID: PMC11155464. https://www.cdc.gov/mmwr/volumes/70/wr/mm7036e1.htm
29. Gregorio Tersalvi, MD, Marco Vicenzi, MD, Davide Calabretta, MD, Luigi Biasco, MD, PhD, Giovanni Pedrazzini, MD, Dario Winterton, MD. Elevated Troponin in Patients with Coronavirus Disease 2019: Possible Mechanisms. Review article| Volume 26, ISSUE 6, P470-475, June 01, 2020. Published:April 18, 2020DOI: https://doi.org/10.1016/j.cardfail.2020.04.009
30. Nascimento JHP, Gomes BFO, Oliveira GMM. Cardiac Troponin as a Predictor of Myocardial Injury and Mortality from COVID-19. Arq Bras Cardiol. 2020 Oct;115(4):667-668. English, Portuguese. doi: 10.36660/abc.20200862. PMID: 33111867. http://abccardiol.org/en/short-editorial/cardiac-troponin-as-a-predictor-of-myocardial-injury-and-mortality-from-covid-19/
31. Choi JY, Lee Y, Park NG, Kim MS, Rhie SJ. Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System. Pharmaceuticals (Basel). 2024 Mar 10;17(3):356. doi: 10.3390/ph17030356. PMID: 38543142; PMCID: PMC10974993. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10974993/
32. Altman NL, Berning AA, Mann SC, Quaife RA, Gill EA, Auerbach SR, Campbell TB, Bristow MR. Vaccination-Associated Myocarditis and Myocardial Injury. Circ Res. 2023 May 12;132(10):1338-1357. doi: 10.1161/CIRCRESAHA.122.321881. Epub 2023 May 11. PMID: 37167355; PMCID: PMC10171307. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10171307/
33. Liao YF, Tseng WC, Wang JK, Chen YS, Chen CA, Lin MT, Lu CW, Wu MH, Chiu SN. Management of cardiovascular symptoms after Pfizer-BioNTech COVID-19 vaccine in teenagers in the emergency department. J Formos Med Assoc. 2023 Aug;122(8):699-706. doi: 10.1016/j.jfma.2022.12.004. Epub 2022 Dec 13. PMID: 36564302; PMCID: PMC9744679. https://doi.org/10.1016/j.jfma.2022.12.004
34. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021 Jun 29. doi: 10.1001/jamacardio.2021.2833. Epub ahead of print. PMID: 34185045. https://jamanetwork.com/journals/jamacardiology/fullarticle/2781601
35. Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, Phelan D, Kim JH, Meeuwisse W, Sills AK, Rowe D, Bogoch II, Smith PT, Baggish AL, Putukian M, Engel DJ. Prevalence of Inflammatory Heart Disease Among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021 Jul 1;6(7):745-752. doi: 10.1001/jamacardio.2021.0565. PMID: 33662103; PMCID: PMC7934073. https://jamanetwork.com/journals/jamacardiology/fullarticle/2777308
36. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020 Nov 1;5(11):1265-1273. doi: 10.1001/jamacardio.2020.3557. Erratum in: JAMA Cardiol. 2020 Nov 1;5(11):1308. PMID: 32730619; PMCID: PMC7385689. https://journals.lww.com/thoracicimaging/fulltext/2021/03000/cardiac_magnetic_resonance_imaging_in_coronavirus.2.aspx
37. Ucar FM, Ozturk C, Yılmaztepe MA. Evaluation of Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio in patients with acute myocarditis. BMC Cardiovasc Disord. 2019 Oct 22;19(1):232. doi: 10.1186/s12872-019-1207-z. PMID: 31640548; PMCID: PMC6805629. https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-019-1207-z
38. Vaccine Adverse Event Reporting System (VAERS) [online]. Available at: https://vaers.hhs.gov
39. Cook KM, Evans G. The National Vaccine Injury Compensation Program. Pediatrics. 2011 May;127 Suppl 1:S74-7. doi: 10.1542/peds.2010-1722K. Epub 2011 Apr 18. PMID: 21502255. https://doi.org/10.1542/peds.2010-1722K
40. Lazarus, Ross et al. Grant Final Report. Grant ID: R18 HS 017045. Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS). Submitted to The Agency for Healthcare Research and Quality (AHRQ). https://digital.ahrq.gov/ahrq-funded-projects/electronic-support-public-health-vaccine-adverse-event-reporting-system
41. Rose, J. 2021, Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System? Science, Public Health Policy & the Law Volume 3:100–129. https://dx.doi.org/https://www.publichealthpolicyjournal.com/
42. NIA Adverse Event and Serious Adverse Event Guidelines [Internet]. [cited 2023 Aug 24]. Available from: https://www.nia.nih.gov/sites/default/files/2018-09/nia-ae-and-sae-guidelines-2018.pdf
43. CDC Covid Data tracker [Internet]. Centers for Disease Control and Prevention; [cited 2023 Aug 24]. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccination-states-jurisdictions
44. FDA approved the first COVID-19 vaccine, Comirnaty (COVID-19 Vaccine, mRNA), which was previously known as Pfizer-BioNTech COVID-19 Vaccine, for the prevention of COVID-19 disease in individuals 16 years of age and older.
45. COVID-19 Vaccines. https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html
46. Miller ER, McNeil MM, Moro PL, Duffy J, Su JR. The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain-Barré syndrome. Vaccine. 2020 Nov 3;38(47):7458-7463. doi: 10.1016/j.vaccine.2020.09.072. Epub 2020 Oct 7. PMID: 33039207. https://doi.org/10.1016/j.vaccine.2020.09.072
47. Walsh EE, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020 Dec 17;383(25):2439-2450. doi: 10.1056/NEJMoa2027906. Epub 2020 Oct 14. PMID: 33053279; PMCID: PMC7583697. https://www.nejm.org/doi/10.1056/NEJMoa2027906?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
48. Polack FP, et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid19 Vaccine. N Engl J Med. 2020 Dec 31;383(27):2603-2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. https://www.nejm.org/doi/10.1056/NEJMoa2110345?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
49. Lei Y, et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE2. Circulation Research. Volume 128, Number 9. https://doi.org/10.1161/CIRCRESAHA.121.318902
50. Vogel G, Couzin-Frankel J. Israel reports link between rare cases of heart inflammation and COVID-19 vaccination in young men [Internet]. Sci. AAAS. 2021 [cited 2021 Jun 6]; Available from: https://www.sciencemag.org/news/2021/06/israel-reports-link-between-rare-cases-heart-inflammation-and-covid-19-vaccination
51. Mansanguan S, Charunwatthana P, Piyaphanee W, Dechkhajorn W, Poolcharoen A, Mansanguan C. Cardiovascular Manifestation of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop Med Infect Dis. 2022 Aug 19;7(8):196. doi: 10.3390/tropicalmed7080196. PMID: 36006288; PMCID: PMC9414075. https://doi.org/10.3390/tropicalmed7080196
52. Buergin N, et al. Sex-specific differences in myocardial injury incidence after COVID-19 mRNA-1273 booster vaccination. Eur J Heart Fail. 2023 Jul 20. doi: 10.1002/ejhf.2978. Epub ahead of print. PMID: 37470105. https://doi.org/10.1002/ejhf.2978
53. Bouchaala A, Nguadi J, Benhlima A, Arfaoui M, Elhamzaoui H, Alilou M. Post-vaccine COVID-19 acute myocarditis: case reports and literature review. Pan Afr Med J. 2023 Apr 20;44:192. doi: 10.11604/pamj.2023.44.192.35425. PMID: 37484597; PMCID: PMC10362684. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10362684/
54. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? Children (Basel). 2021 Jul 18;8(7):607. doi: 10.3390/children8070607. PMID: 34356586; PMCID: PMC8305058. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8305058/
55. Cho JY, et al. COVID-19 vaccination-related myocarditis: a Korean nationwide study. Eur Heart J. 2023 Jun 25;44(24):2234-2243. doi: 10.1093/eurheartj/ehad339. PMID: 37264895; PMCID: PMC10290868. https://doi.org/10.1093/eurheartj/ehad339
56. Manu P. Fatal Myocarditis After COVID-19 Vaccination: Fourteen Autopsy-Confirmed Cases. Am J Ther. 2023 May 1;30(3):e259-e260. doi: 10.1097/MJT.0000000000001631. Erratum in: Am J Ther. 2023 Sep-Oct 01;30(5):e507. doi: 10.1097/MJT.0000000000001658. PMID: 37278705. https://journals.lww.com/americantherapeutics/citation/2023/06000/fatal_myocarditis_after_covid_19_vaccination_.9.aspx
57. Rose J, Hulscher N, McCullough PA. Determinants of COVID-19 vaccine-induced myocarditis. Ther Adv Drug Saf. 2024 Jan 27;15:20420986241226566. doi: 10.1177/20420986241226566. PMID: 38293564; PMCID: PMC10823859. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10823859/
58. There are a total of 12 influenza products by type administered in the U.S. between 2018-2020 (FLU3, FLU4, FLUA3, FLUA4, FLUC3, FLUC4, FLUN3, FLUN4, FLUR3, FLUR4, FLUX, FLUX(H1N1). https://vaers.hhs.gov
59. https://www.meddra.org/basics
60. GitHub. owid/covid-19-data. Data on COVID-19 (coronavirus) by Our World in Data. Updated daily by Our World in Data. [Accessed: 12 Aug 2023]. Available from: https://github.com/owid/covid-19-data/tree/master/public/data
61. Walach Harald, Klement Rainer J, Aukema Wouter. The Safety of COVID-19 Vaccinations - Should We Rethink the Policy? Sci, Publ Health Pol & Law, 3 (2021), pp. 87-99. https://publichealthpolicyjournal.com/the-safety-of-covid-19-vaccinations-should-we-rethink-the-policy/
62. Halasa NB, Olson SM, Staat MA, et al. Effectiveness of Maternal Vaccination with mRNA COVID-19 Vaccine During Pregnancy Against COVID-19–Associated Hospitalization in Infants Aged <6 Months — 17 States, July 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:264–270. DOI: http://dx.doi.org/10.15585/mmwr.mm7107e3
63. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#pregnancy-fertility
64. Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Fail. 2024 Jan 14. doi: 10.1002/ehf2.14680. Epub ahead of print. PMID: 38221509. https://doi.org/10.1002/ehf2.14680
65. Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J. 'Spikeopathy': COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines. 2023 Aug 17;11(8):2287. doi: 10.3390/biomedicines11082287. PMID: 37626783; PMCID: PMC10452662. https://doi.org/10.3390/biomedicines11082287
66. Gill JR, Tashjian R, Duncanson E. Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose. Arch Pathol Lab Med. 2022 Aug 1;146(8):925-929. doi: 10.5858/arpa.2021-0435-SA. PMID: 35157759. https://doi.org/10.5858/arpa.2021-0435-SA
67. Mungmunpuntipantip R, Wiwanitkit V. Autopsy Histopathologic Cardiac Findings Following the Second COVID-19 Vaccine Dose. Arch Pathol Lab Med. 2022 Dec 1;146(12):1432. doi: 10.5858/arpa.2022-0171-LE. PMID: 36445987. https://doi.org/10.5858/arpa.2022-0171-LE
68. Suzuki H, Ro A, Takada A, Saito K, Hayashi K. Autopsy findings of post-COVID-19 vaccination deaths in Tokyo Metropolis, Japan, 2021. Leg Med (Tokyo). 2022 Nov;59:102134. doi: 10.1016/j.legalmed.2022.102134. Epub 2022 Aug 20. PMID: 36037554; PMCID: PMC9392553. https://doi.org/10.1016/j.legalmed.2022.102134
69. Pezzullo AM, Axfors C, Contopoulos-Ioannidis DG, Apostolatos A, Ioannidis JPA. Age-stratified infection fatality rate of COVID-19 in the non-elderly population. Environ Res. 2023 Jan 1;216(Pt 3):114655. doi: 10.1016/j.envres.2022.114655. Epub 2022 Oct 28. PMID: 36341800; PMCID: PMC9613797. https://doi.org/10.1016/j.envres.2022.114655
70. Pierce CA, Sy S, Galen B, Goldstein DY, Orner E, Keller MJ, Herold KC, Herold BC. Natural mucosal barriers and COVID-19 in children. JCI Insight. 2021 May 10;6(9):e148694. doi: 10.1172/jci.insight.148694. PMID: 33822777; PMCID: PMC8262299. https://insight.jci.org/articles/view/148694
71. Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. Vitamin D and COVID-19: where are we now? Postgrad Med. 2023 Apr;135(3):195-207. doi: 10.1080/00325481.2021.2017647. Epub 2021 Dec 27. PMID: 34886758; PMCID: PMC8787834. https://doi.org/10.1080/00325481.2021.2017647
72. Tabatabaeizadeh SA. Zinc supplementation and COVID-19 mortality: a meta-analysis. Eur J Med Res. 2022 May 23;27(1):70. doi: 10.1186/s40001-022-00694-z. PMID: 35599332; PMCID: PMC9125011. https://eurjmedres.biomedcentral.com/articles/10.1186/s40001-022-00694-z
73. McCullough PA, et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med. 2021 Jan;134(1):16-22. doi: 10.1016/j.amjmed.2020.07.003. Epub 2020 Aug 7. PMID: 32771461; PMCID: PMC7410805. https://doi.org/10.1016/j.amjmed.2020.07.003
74. Yuan Y, Jiao B, Qu L, Yang D, Liu R. The development of COVID-19 treatment. Front Immunol. 2023 Jan 26;14:1125246. doi: 10.3389/fimmu.2023.1125246. PMID: 36776881; PMCID: PMC9909293. https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2023.1125246/full
75. Majumder J, Minko T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021 Jan 5;23(1):14. doi: 10.1208/s12248-020-00532-2. PMID: 33400058; PMCID: PMC7784226. https://link.springer.com/article/10.1208/s12248-020-00532-2
76. Nakagawa A, Nakamura N, Torii S, Goto S. Acute pulmonary hypertension due to microthrombus formation following COVID-19 vaccination: a case report. Eur Heart J Case Rep. 2023 Jul 26;7(8):ytad353. doi: 10.1093/ehjcr/ytad353. PMID: 37559783; PMCID: PMC10409304. https://academic.oup.com/ehjcr/article/7/8/ytad353/7231708?login=false
77. Bekal S, Husari G, Okura M, Huang CA, Bukari MS. Thrombosis Development After mRNA COVID-19 Vaccine Administration: A Case Series. Cureus. 2023 Jul 4;15(7):e41371. doi: 10.7759/cureus.41371. PMID: 37546104; PMCID: PMC10400017. https://www.cureus.com/articles/166699-thrombosis-development-after-mrna-covid-19-vaccine-administration-a-case-series#!/
78. Kim EJ, Yoo SJ. Pulmonary Embolism after Vaccination with the COVID-19 Vaccine (Pfizer, BNT162b2): A Case Report. Vaccines (Basel). 2023 Jun 7;11(6):1075. doi: 10.3390/vaccines11061075. PMID: 37376463; PMCID: PMC10303489. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10303489/
79. Castruita JAS, Schneider UV, Mollerup S, Leineweber TD, Weis N, Bukh J, Pedersen MS, Westh H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS. 2023 Mar;131(3):128-132. doi: 10.1111/apm.13294. Epub 2023 Jan 29. PMID: 36647776; PMCID: PMC10107710. https://doi.org/10.1111/apm.13294
80. Yonker LM et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation. 2023 Mar 14;147(11):867-876. doi: 10.1161/CIRCULATIONAHA.122.061025. Epub 2023 Jan 4. PMID: 36597886; PMCID: PMC10010667. https://doi.org/10.1161/CIRCULATIONAHA.122.061025
81. Baumeier C, et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int J Mol Sci. 2022 Jun 22;23(13):6940. doi: 10.3390/ijms23136940. PMID: 35805941; PMCID: PMC9266869. https://doi.org/10.3390/ijms23136940
82. McPhillips HA, Davis RL, Marcuse EK, Taylor JA. The Rotavirus Vaccine's Withdrawal and Physicians' Trust in Vaccine Safety Mechanisms. Arch Pediatr Adolesc Med. 2001;155(9):1051–1056. doi:10.1001/archpedi.155.9.1051. https://jamanetwork.com/journals/jamapediatrics/fullarticle/191036
83. Buonocore SM, van der Most RG. Narcolepsy and H1N1 influenza immunology a decade later: What have we learned? Front Immunol. 2022 Oct 12;13:902840. doi: 10.3389/fimmu.2022.902840. PMID: 36311717; PMCID: PMC9601309. https://doi.org/10.3389/fimmu.2022.902840
84. Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine--United States, June 2005-September 2006. MMWR Morb Mortal Wkly Rep. 2006 Oct 20;55(41):1120-4. Erratum in: MMWR Morb Mortal Wkly Rep. 2006 Nov 3;55(43):1177. PMID: 17060898
85. Urszula Grzybowska-Chlebowczyk, Monika Kałużna-Czyż, Barbara Kalita, Katarzyna Gruszczyńska, Sabina Więcek, Monika Dębowska, Wojciech Chlebowczyk, Halina Woś, Intussusception as a complication of rotavirus infection in children, Pediatria Polska, Volume 90, Issue 6, 2015, Pages 464-469, ISSN 0031-3939, https://doi.org/10.1016/j.pepo.2015.08.004
86. Pradhan SK, Dash M, Ray RK, Mohakud NK, Das RR, Satpathy SK, Chaudhury J, Prusty JB, Padhi PS, Mohanty SK, Das M, Reddy N S, Nayak MK. Childhood Intussusception after Introduction of Indigenous Rotavirus Vaccine: Hospital-Based Surveillance Study from Odisha, India. Indian J Pediatr. 2021 Mar;88(Suppl 1):112-117. doi: 10.1007/s12098-020-03627-y. Epub 2021 Feb 5. PMID: 33544368. https://link.springer.com/article/10.1007/s12098-020-03627-y
87. Hernán MA, Jick SS, Olek MJ, Jick H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology. 2004 Sep 14;63(5):838-42. doi: 10.1212/01.wnl.0000138433.61870.82. PMID: 15365133. https://doi.org/10.1212/01.WNL.0000138433.61870.82
88. Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020 Jun;109(6):1088-1095. doi: 10.1111/apa.15270. Epub 2020 Apr 14. PMID: 32202343; PMCID: PMC7228328. https://doi.org/10.1111/apa.15270
89. Yu CK, Tsao S, Ng CW, Chua GT, Chan KL, Shi J, Chan YY, Ip P, Kwan MY, Cheung YF. Cardiovascular Assessment up to One Year After COVID-19 Vaccine-Associated Myocarditis. Circulation. 2023 Aug;148(5):436-439. doi: 10.1161/CIRCULATIONAHA.123.064772. Epub 2023 Jul 31. PMID: 37523760; PMCID: PMC10373639. https://doi.org/10.1161/CIRCULATIONAHA.123.064772
90. Menezes RG, Monteiro FN. Forensic Autopsy. [Updated 2023 Sep 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539901/
91. Halma MTJ, Rose J, Lawrie T. The Novelty of mRNA Viral Vaccines and Potential Harms: A Scoping Review. J. 2023; 6(2):220-235. https://doi.org/10.3390/j6020017. https://doi.org/10.3390/j6020017
92. COVID-19 Modified mRNA “Vaccines” Part 1: Lessons Learned from Clinical Trials, Mass Vaccination, and the Bio-Pharmaceutical Complex. (2024). International Journal of Vaccine Theory, Practice, and Research , 3(2), 1112-1178. https://doi.org/10.56098/fdrasy50
93. United States Government Accountability Office. OPERATION WARP SPEED-Accelerated COVID-19 Vaccine Development Status and Efforts to Address Manufacturing Challenges Feb, 2021. Available from: https://www.gao.gov/assets/gao-21-319.pdf
94. Worse Than the Disease? Reviewing Some Possible Unintended Consequences of the mRNA Vaccines Against COVID-19. (2021). International Journal of Vaccine Theory, Practice, and Research , 2(1), 38-79. https://doi.org/10.56098/ijvtpr.v2i1.23 (Original work published 2021)



No comments:
Post a Comment