PCR Fraud
The fraudulent use of the PCR process to "diagnose" disease must be STOPPED. You always have the right to say NO. Defend your bodily autonomy and say NO to the fraudulent use of the PCR.
Share this link: PCRfraud.com
Dedicated to the memory of Kary Mullis, who received the Nobel Prize for his work on the PCR process, on the fifth anniversary of his death on August 7, 2019.
This article provides access to legal decisions, official government documents and peer-reviewed scientific reports to help support YOU in your efforts to stand up in defense of your unalienable rights.
Please watch ALL the videos below:
https://www.youtube.com/watch?v=LAPqYpwSyXI
Backup link: https://www.brighteon.com/5b977d50-1015-490e-8d10-0bbb8397c767
https://rumble.com/v5a2npp-pcr-fraud.html
Yes, I know that the video above is one hour long.
Please watch it.
It is very important!
https://tntvideo.podbean.com/e/james-roguski-on-weekends-with-jason-olbourne-04-august-2024
https://rumble.com/v5a3e2c-pcr-fraud-tribute-to-truth-sue-the-who.html
Support Interest of Justice’s efforts to Sue the WHO:
SueTheWHO.org/PCRfraud
For decades, public health policies around the globe have been based on a scam:
The use of PCR (Polymerase Chain Reaction) “tests” to create wildly inflated “case counts” that authorities can then cite to impose lockdowns, closings, quarantines, mask and vaccine mandates is fraudulent.
Public health policies based upon a meaningless count of positive PCR “tests” are not justifiable because PCR “testing” CANNOT determine whether anyone is currently infected with a particular pathogen, whether they are currently capable of transmitting the pathogen to others or whether any specific pathogen is the cause of any disease or symptoms.
The fraudulent use of the RT-PCR process as a diagnostic “test” clearly led to a large number of false positive results that greatly inflated the numbers of asymptomatic “cases” of COVID-19.
Because PCR isn’t a test for infection or illness, all “positive” results can be seen as false positives. The entire process is fraudulent.
The fraud of the COVID-19 PCR was called out by eminent scientists very early on. While it may be too late to stop the mass “testing” for COVID-19, it’s not too late to stand up against its continued use.
Such fraudulent testing has been used to trigger fear in the past, and it will lead to future “casedemics” unless vast numbers of people stand up for their unalienable rights and REFUSE to be tricked by this ongoing fraud.
It’s worth taking the time to truly understand how this scam has been perpetrated because once you understand it, you can help to make sure it never happens again.
Please download the PDFs below, print them out, read them, use them to defend your rights, and share them far and wide.
Before anyone ever attempts to coerce you to submit to any form of health related “test,” please read this article, watch the videos and know the facts so that you can defend your bodily autonomy and property rights.
English Version:
Notice And Statement Of Facts
1.18MB ∙ PDF file
Spanish Version:
Aviso Y Declaracion De Hechos
298KB ∙ PDF file
Steger-Kammerer PCR Factsheet (English):
Steger Kammerer Pcr Factsheet English
300KB ∙ PDF file
Steger-Kammerer PCR Factsheet (German):
Steger Kammerer Pcr Factsheet German
279KB ∙ PDF file
https://www.mwgfd.org/2024/08/infoblatt-pcr/
READ THE FOLLOWING ARTICLES:
Statements by Experts:
These tests are not 100% sensitive or specific. If you have 1% of your population infected and you have a test that’s only 99% specific, that means that when you find a positive, 50% of the time it will be a real positive and 50% of the time it won’t be.
Deborah Birx (former White House Coronavirus Response Coordinator)
PCR doesn’t measure replication competent virus. It measures viral particles’ nucleic acid. So, in other words, I could be infected, have cleared the replication competent virus from me, but I can continue to be positive with the PCR for several days after recovering and not being transmissible at all.
But the very fact that it’s positive, for as the CDC Director said, for several days and even weeks later, it doesn’t give you any indication of whether or not you’re transmissible.
The only way you can tell if it’s transmissible, if you can show that there really is live, replication virus in you, and the tests don’t measure that.
Anthony Fauci (former head of NIAID)
It is claimed that PCR mass tests represent a suitable tool for surveilling viral dissemination and population health, but this is NOT TRUE. Testing for virus subtypes may be of scientific interest, but has no relevance in a routine clinical setting.
Symptoms of respiratory tract infections may be caused by a variety of different viruses and even bacteria, fungi or protozoa, alone or in combination.
Any positive PCR test result considered on its own cannot prove the presence of a replication-competent, infectious virus. Even when performed properly, PCR can by no means prove that an infectious virus with an intact genome is present in an analyzed sample.
PCR-positive individuals do not necessarily transmit or even carry an intact virus.
Klaus Steger (Co-author of the Corman-Drosten Review)
A PCR test - even if performed correctly - cannot provide any information on whether a person is infected with an active pathogen or not.
PCR in all forms (PCR, RT-PCR, or RT-qPCR) is only suitable for detecting genome fragments and is not a suitable reliable (and approved) diagnostic tool for detecting intact, infectious (replicable) SARS-CoV-2 viruses.
PCR must not and can never be the sole diagnostic tool for a possible disease.
Ulrike Kämmerer (Co-author of the Corman-Drosten Review)
If you're testing in a population that doesn't have very much COVID, you'll get false positives almost half the time. The person actually doesn't have COVID. They have something else. They may have nothing.
Dr. Barbara Yaffe, Associate Chief Medical Officer of Health, Ontario, Canada
Even if there were respiratory viruses that cause acute illnesses such as colds and flu, and even if PCR was conducted carefully, finding a partial sequence is still meaningless. It doesn’t say you’re ill nor that you have been ill or that you’re contagious. Not one element of this charade is even capable of yielding what the authorities claim for it. All the “positive results” are false positives. Do not get tested. You validate one of the Big Club’s prime control mechanisms by doing so.
Michael Yeadon (former Pfizer executive)
PCR can test for the presence of viral RNA. PCR testing cannot test for viral infectiousness or illness.
-Kevin McKernan (CSO and Founder of Medicinal Genomics)
A binary Yes/No approach to the interpretation RT-PCR unvalidated against viral culture will result in false positives with possible segregation of large numbers of people who are no longer infectious and hence not a threat to public health.
-Carl Heneghan (Director of the University of Oxford's Centre for Evidence-Based Medicine)
We have been unable to find any data on the operational false positive and false negative rates in the UK COVID-19 RT-PCR testing programme. The UK operational false positive rate is unknown.
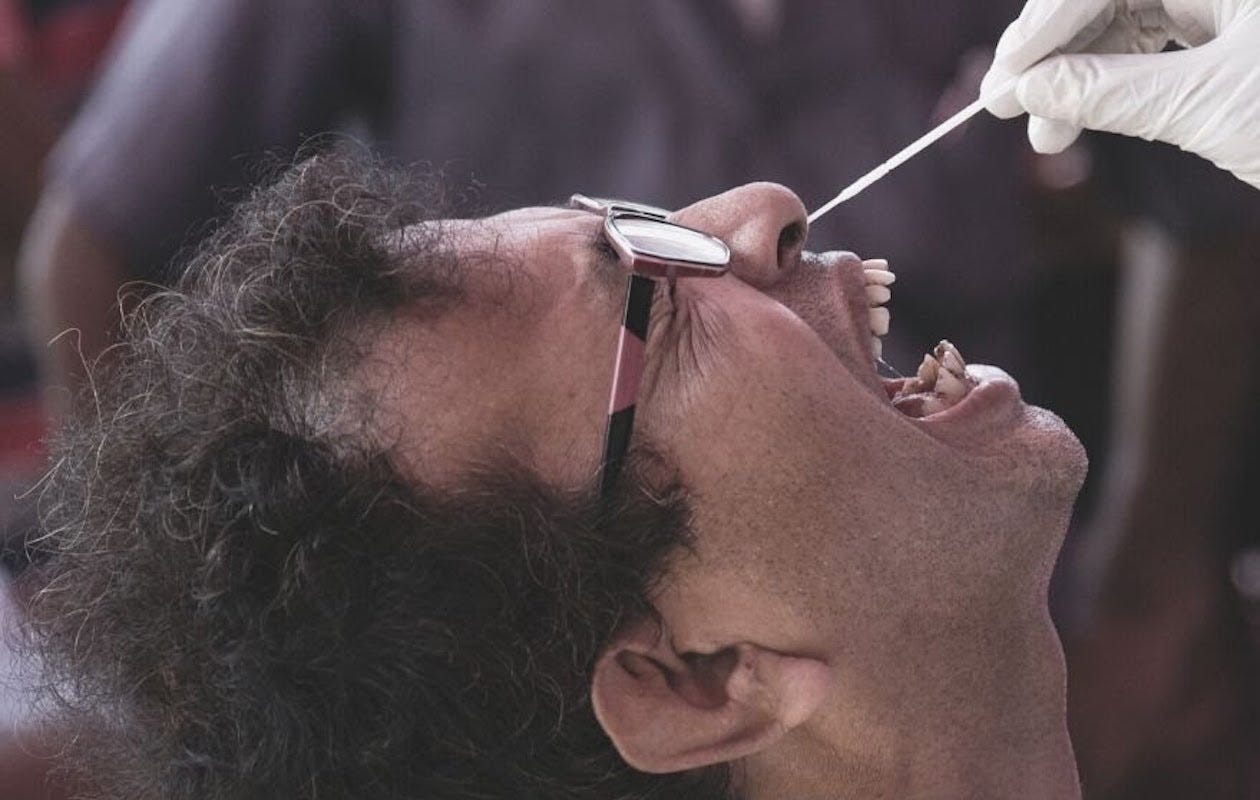
Have you ever been violated in the manner pictured below?
Were you told that such a procedure was “required” in order to determine whether or not you had COVID-19?
You were lied to.
CONSIDER THIS:
When the RT-PCR process is run through over 40 cycles, it can “amplify” one molecule into over one trillion molecules. [Two to the fortieth power equals 1,099 ,511, 627, 776.]
Imagine if the RT-PCR process were to be adapted to test for alcohol in one’s blood.
Nearly everyone would test “positive” for alcohol in their blood at 0.00000000008%, but that would not mean that they were “drunk.”
Similarly, if one molecule of a snippet of genetic material is scraped from the inside of your nasal cavity, a “positive” result does not mean that you are “ill,” or “contagious.”
Using the RT-PCR process to declare that someone has an “asymptomatic case” of a disease is absolutely absurd.
No one needs a “test” to determine whether or not they are ill.
PUT THEM ON NOTICE!
Please download the PDFs below, print them out, read them, use them to defend your rights and share them far and wide.
PROACTIVELY send the printed version of the Notice and Statement of Facts PDF to EVERY…
Politician
Government/public health official
Hospital administrator
Doctor/health care practitioner
Media outlet
Stand up for your rights and refuse to allow yourself or anyone else to ever be violated again.
Before anyone ever attempts to coerce you to submit to any form of health related “test,” please read this article, watch the videos and know the facts so that you can defend your bodily autonomy and property rights.
English Version:
Notice And Statement Of Facts
1.18MB ∙ PDF file
Spanish version:
Aviso Y Declaracion De Hechos
298KB ∙ PDF file
Steger-Kammerer PCR Factsheet (English):
Steger Kammerer Pcr Factsheet English
300KB ∙ PDF file
Steger-Kammerer PCR Factsheet (German):
Steger Kammerer Pcr Factsheet German
279KB ∙ PDF file
https://www.mwgfd.org/2024/08/infoblatt-pcr/
Copy the text of the “Notice of Facts” below and feel free to edit and adapt it to suit your specific needs.
Notice of Facts
You are hereby notified that the facts included in this document clearly show that the use of the RT-PCR process as a diagnostic “test” is a fraudulent act.
You are also hereby notified that any attempt on your part to coerce, intimidate, mandate, force or obligate me in any way to undergo such testing will be viewed as an attempt on your part to engage in an act of fraud and a potential violation of my bodily autonomy and my right of informed dissent to reject any and all tests and treatments.
Before you continue to attempt to require anyone to undergo RT-PCR “testing” you must be aware that every man, woman and child always has the unalienable right to REFUSE to undergo any form of health related treatment, including (and especially) inaccurate and inappropriate procedures that fail to provide accurate and verifiable diagnoses.
Simply stated, the RT-PCR “test” does not “work,” because RT-PCR is actually a laboratory manufacturing process that was never designed to be used as a test to diagnose disease. This evidence includes court decisions from Portugal, Germany and Canada, official government documents and numerous peer-reviewed articles that have been published in various medical journals.
The RT-PCR process does not (and cannot) detect viable, infectious viruses.
The RT-PCR process does not (and cannot) diagnose disease, contagiousness or infectivity.
The RT-PCR process does not (and cannot) determine whether any specific pathogen is the actual cause of a disease or any collection of symptoms.
The RT-PCR process can and does result in false positive results.
Failure to acknowledge the frequency of false positive test results and the subsequent misguided decisions based on these fraudulent results have led to a plethora of unjustified consequences:
Unnecessary contact tracing and fraudulent “testing” has led to the wrongful isolation and quarantine of men, women and children.
Social distancing, mask mandates and loneliness have had negative consequences on human relations.
People have received inaccurate diagnoses and inappropriate medical treatment.
Serious diagnoses were wrongly given to terrorized people who were actually in good health.
Unprecedented financial, mental, emotional and psychological stress has been inflicted upon millions of people.
Delays in surgical or other procedures and prolonged hospital stays have been experienced.
Many employees and small business owners have lost their means of earning a living.
Education, travel, dining, leisure and other social activities were curtailed.
Clinical trials based on RT-PCR “testing” are meaningless.
Epidemiological statistics have been falsified, leading to exaggerated prevalence, hospitalization, and death rates.
You have 30 days to provide a point by point rebuttal of the following information. Unless and until such a point by point rebuttal has been provided, it shall stand as accepted fact that the use of the RT-PCR process as a diagnostic “test” to determine “cases” of disease is a fraudulent use of the technology and that such testing should never be required of any man, woman, child or of their animals or property.
Please watch the 13 minute video summary of the Statement of Facts:
https://rumble.com/v5a288t-pcr-fraud-details.html
Statement of Facts:
On November 11, 2020, the Court of Appeal of Lisbon (Portugal) ruled that the RT-PCR process shows itself to be unable to determine beyond reasonable doubt that such positivity corresponds, in fact, to the infection of a person by the SARS-CoV-2 virus.
On April 8, 2021, the Weimar (Germany) Family Court ruled that, effective immediately, two Weimar schools are prohibited from requiring students to wear mouth-to-nose coverings of any kind (especially qualified masks such as FFP2 masks), impose minimum AHA distances on them, and/or participate in SARS-CoV-2 rapid testing.
On June 26, 2024, the Ontario (Canada) Court of Justice ruled that people were not obligated to submit to invasive “testing” such as the nasopharyngeal swab used to collect samples for the RT-PCR process.
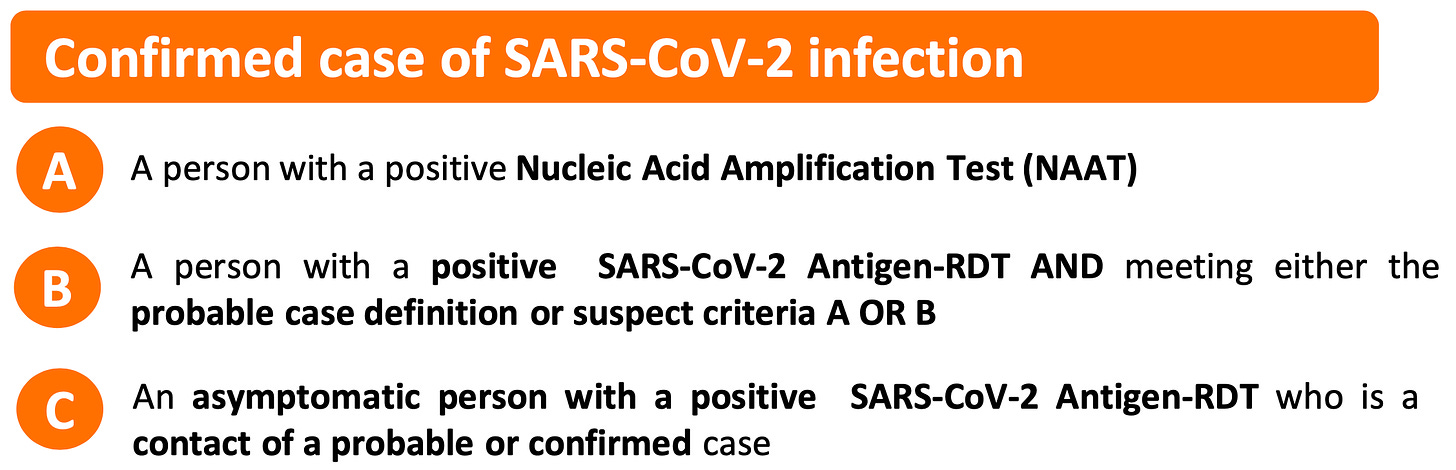
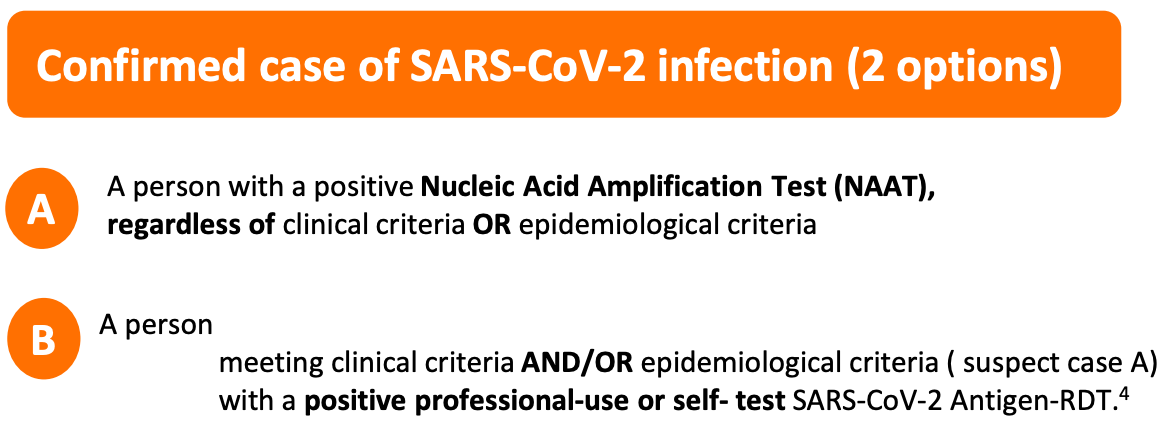
The World Health Organization has defined a COVID-19 “case” as a positive PCR test. The reliance on the RT-PCR process as the sole requirement to determine a COVID-19 “case” without a differential diagnosis based on clinical observations was essentially unheard of prior to 2020.
The RT-PCR process defined in the original “test” (Corman-Drosten) was NOT based upon an isolated virus. It was based upon a genetic sequence that was compiled in a computer (in silico).
The RT-PCR process is NOT a test that can diagnose illness or contagiousness. Its was never intended to be a diagnostic tool. The PCR process merely creates copies of genetic material found in a sample. The detection of certain molecules via the RT-PCR process does NOT provide evidence of disease or contagiousness. The presence of nucleic acids alone should not be used to infer disease, infection, viral shedding or potential contagiousness.
By design, the initial steps in the RT-PCR process destroy the source material so that the RT-PCR process CANNOT possibly measure intact viruses. A so-called “positive” result does not ensure the presence of individual virions.
The potential for false positives from use of the RT-PCR process is enormous. Even if the specificity of a “test” is 99%, if the prevalence of infection in the community is 1/100, then the “test” will return a false positive result 50% of the time.
There is ample evidence that any claims of a “positive” result obtained by running the RT-PCR process through more than 24 cycles are actually false positives.
There is no proof that the nucleic acid sequences that are utilized in any of the various RT-PCR “tests” identify the presence of a viable pathogen that causes COVID-19. An unknown number of other pathogens that are NOT being tested for may also be present. Even if the RT-PCR process identifies the existence of genetic remnants of SARS-CoV-2, it does not rule out the possibility that something else may be the actual cause of disease.
Detailed documentation supporting the top 10 things to know about PCR “tests” is listed below.
1
On November 11, 2020, the Court of Appeal of Lisbon (Portugal) ruled that the RT-PCR process shows itself to be unable to determine beyond reasonable doubt that such positivity corresponds, in fact, to the infection of a person by the SARS-CoV-2 virus.
Court of Appeal of Lisbon
The Lisbon Court of Appeal upheld a lower court's decision in support of the writ of habeas corpus that had been filed by the four German travelers and ruled that the Azores Regional Health Authority had violated both Portuguese and international law by confining the four German travelers to a hotel. The judges ruled that only a doctor can “diagnose” someone with a disease, and they were critical of the fact that the four German travelers were apparently never assessed by a medical doctor.
II. The request made would also be manifestly unfounded because:
A. Prescription and diagnosis are medical acts, the sole responsibility of a doctor, registered with the Medical Association. Thus, the prescription of auxiliary diagnostic methods (as is the case with viral infection detection tests), as well as the diagnosis of the existence of a disease, in relation to any and all people, is a matter that cannot be carried out by Law , Resolution, Decree, Regulation or any other normative means , as these are acts that our legal system reserves to the exclusive competence of a doctor, given that he, when advising his patient, must always try to obtain his informed consent.
B. In the case at hand, there is no indication or proof that such a diagnosis was actually carried out by a professional qualified under the terms of the Law and who had acted in accordance with good medical practice. In fact, what follows from the facts as established is that none of the applicants was even seen by a doctor, which is frankly inexplicable, given the alleged severity of the infection.
C. The only element that appears in the proven facts, in this regard, is the carrying out of RT-PCR tests, one of which showed a positive result in relation to one of the applicants.
D. In view of the current scientific evidence, this test alone proves to be incapable of determining, without a reasonable margin of doubt, that such positivity corresponds, in fact, to a person's infection with the SARS-CoV-2 virus, for several reasons, of which we highlight two:
Because this reliability depends on the number of cycles that make up the test;
Because this reliability depends on the amount of viral load present.
III . Any diagnosis or any act of health surveillance... carried out without prior medical observation of the patients and without the intervention of a doctor registered with the OM (who would carry out the assessment of their signs and symptoms, as well as examinations that he considered appropriate to his condition), violates Regulation no. 698/2019, of 5.9, as well as the provisions of article 97 of the Statute of the Medical Association, being liable to constitute the crime of functions, e.g. and p. by article 358 al.b), of the C.Penal.
IV. Any person or entity that issues an order, the content of which leads to the deprivation of physical, ambulatory, or other people's freedom (whatever nomenclature this order assumes: confinement, isolation, quarantine, prophylactic protection, health surveillance, etc.), which does not comply with the legal provisions, namely the provisions of article 27 of the CRP , you will be carrying out an illegal detention.
2
On April 8, 2021, the Weimar (Germany) Family Court ruled that, effective immediately, two Weimar schools are prohibited from requiring students to wear mouth-to-nose coverings of any kind (especially qualified masks such as FFP2 masks), impose minimum AHA distances on them, and/or participate in SARS-CoV-2 rapid testing.
Weimar Family Court (in the federal state of Thuringia)
The unsuitability of PCR tests and rapid tests for measuring the incidence of infection.
Regarding the PCR test, the court writes: “Already the expert Prof. Dr. med. Kappstein points out in her expert opinion that only genetic material can be detected with the PCR test used, but not whether the RNA originates from viruses capable of infection and thus capable of replication (= reproduction).
Also the expert Prof. Dr. rer. biol. hum. Kämmerer also confirms in her expert opinion on molecular biology that a PCR test – even if it is carried out correctly – cannot provide any information on whether a person is infected with an active pathogen or not.
This is because the test cannot distinguish between “dead” matter*, e.g. a completely harmless genome fragment as a remnant of the body’s own immune system’s fight against a cold or flu (such genome fragments can still be found many months after the immune system has “dealt with” the problem) and “living” matter, i.e. a “fresh” virus capable of reproducing.
So, even if everything is done “correctly” when performing the PCR including all preparatory steps (PCR design and establishment, sample collection, preparation and PCR performance), and the test is positive, i.e.: detects a genome sequence which may also exist in one or even the specific “Corona” virus (SARS-CoV-2), this does not mean under any circumstances that the person who was tested positive is infected with a replicating SARS-CoV-2 and consequently infectious = dangerous for other persons.
Rather, for the determination of an active infection with SARS-CoV-2, further, and specifically diagnostic methods such as the isolation of replicable viruses must be used.
The expert points out that, according to unanimous scientific opinion, all “positive” results that are only detected after a cycle of 35 have no scientific (i.e.: no evidence-based) basis. In the ct range 26-35, the test can only be considered positive if matched with viral culture. In contrast, the RT-qPCR test for the detection of SARS-CoV-2, which was propagated worldwide with the help of the WHO, was (and following it all other tests based on it as a blueprint) set to 45 cycles without defining a ct value for “positive”.
Finally, the reviewer points out that the low specificity of the tests causes a high rate of false positives, which result in unnecessary personnel (quarantine) and societal (e.g., schools closed, “outbreak notifications”) consequences until they turn out to be false alarms. The error effect, i.e., a high number of false positives, is particularly strong in tests on symptomless individuals.
It remains to be stated that the PCR test used, as well as the antigen rapid tests, as proven by the expert opinion, are in principle not suitable for the detection of an infection with the virus SARS-CoV-2.
In addition, the described and other sources of error listed in the expert opinion with serious effects, so that an adequate determination of the infection with SARS-CoV-2 in Thuringia (and nationwide) is not rudimentarily available.
3
June 26, 2024, the Ontario (Canada) Court of Justice ruled that people were not obligated to submit to invasive “testing” such as the nasopharyngeal swab used to collect samples for the RT-PCR process.
Ontario Court of Justice
Ms. Fernando took an airplane flight to her home in Mississauga, arriving at Pearson Airport on April 9th, 2022. She was apparently vaccinated, but she refused the COVID test, which was randomly selected to be performed on her. In particular, she was sked... to undergo a nasal swab COVID-19 test, and she refused.
Ms. Fernando was convicted at trial of failing to comply with an order under Section 58 of the Quarantine Act (the “Act”) and fined $5,000 with additional charges, taking it to a fine of $6,255. She appeals now to this Court.
The argument, simply put, is that the Act did not authorize a screening officer to use a screening test which involved the entry into the traveller's body of an instrument or other foreign body.
The screening test that Mr. Roxas proposed involved the insertion of a nasal swab into Ms. Fernando's nasal cavity, contrary to Section 14 of the Quarantine Act.
The relevant provisions are as follows, quoting Section 14 of the Quaranting Act:
Screening Technology
14(1) Any qualified person authorized by the Minister may, to determine whether a traveller has a communicable disease or symptoms of one, use any screening technology authorized by the Minister that does not involve the entry into the traveller's body of any instrument or other foreign body.
The prosecution raised the point that perhaps the insertion into the nasal cavity did not involve the entry into the body. I disagree. The insertion of a nasal swab into the nasal cavity is most definitely an insertion into the body.
I do decide that the nasal swab test, which the screening officer in this case required or demanded Ms. Fernando submit to, was an unlawful requirement or demand. Ms. Fernando's refusal to comply with the requirement or demand was lawful on her part. Because the requirement or demand made of her by the screening officer was not lawful, Ms. Fernando should not have been found guilty by the Justice of the Peace.
I am reversing the Justice of the Peace's decision and entering a finding of not guilty.
Read this article:
4
The World Health Organization defined a COVID-19 “case” as a positive PCR test. The reliance on the RT-PCR process as the sole requirement to determine a COVID-19 “case” without a differential diagnosis based on clinical observations was essentially unheard of prior to 2020.
March 20, 2020
Confirmed case
A person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms.
December 16, 2020
WHO COVID-19 Case definition
July 22, 2022 to present
https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1
International Journal of Vaccine Theory, Practice, and Research
For the first time in medical history, a laboratory assay (RT-PCR) was used as the sole criterion to diagnose a disease (COVID-19) and to define infectivity of a virus (SARS-CoV-2) without rating clinical symptoms and proof of replication-competent virus to justify implementing population-wide, untested interventions.
Unnecessary quarantine of healthy individuals, as well as lockdowns and atrocious collateral damage on societies and economies worldwide due to a high number of false-positive “PCR-cases.” Otherwise, infectious symptomatic individuals were given a false sense of security by false-negative test results, which could lead to COVID-19 clusters. Both our results and literature data confirm that validation of any PCR-based diagnostic test by sequencing is mandatory on a regular basis. To prevent future misconduct, science needs a reality check and must re-initiate the scientific dialogue and liberate itself from political influence and dogma.
International Journal of Vaccine Theory, Practice, and Research
PCR testing has been erroneously chosen as the gold standard for diagnosing COVID-19 infection and disease, even if it has never been validated, nor standardized. The symptoms of COVID-19 disease cannot be specified, because they can be anything, everything, and nothing at all according to the authorities. They range from clinically observable symptoms likely to lead to death to no symptoms at all — from near death to complete health. All the foregoing shows the entire scope of COVID-19 diagnostic science is flawed.
5
The RT-PCR process defined in the original “test” (Corman-Drosten) was NOT based upon an isolated virus. It was based upon a genetic sequence that was compiled in a computer (in silico).
The Corman-Drosten paper contains the following specific errors:
There exists no specified reason to use these extremely high concentrations of primers in this protocol. The described concentrations lead to increased nonspecific bindings and PCR product amplifications, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
Six unspecified wobbly positions will introduce an enormous variability in the real world laboratory implementations of this test; the confusing nonspecific description in the Corman-Drosten paper is not suitable as a Standard Operational Protocol making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
The test cannot discriminate between the whole virus and viral fragments. Therefore, the test cannot be used as a diagnostic for intact (infectious) viruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus and make inferences about the presence of an infection.
A difference of 10° C with respect to the annealing temperature Tm for primer pair1 (RdRp_SARSr_F and RdRp_SARSr_R) also makes the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
A severe error is the omission of a Ct value at which a sample is considered positive and negative. This Ct value is also not found in follow-up submissions making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
The PCR products have not been validated at the molecular level. This fact makes the protocol useless as a specific diagnostic tool to identify the SARS-CoV-2 virus.
The PCR test contains neither a unique positive control to evaluate its specificity for SARS-CoV-2 nor a negative control to exclude the presence of other coronaviruses, making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
The test design in the Corman-Drosten paper is so vague and flawed that one can go in dozens of different directions; nothing is standardized and there is no SOP. This highly questions the scientific validity of the test and makes it unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
Most likely, the Corman-Drosten paper was not peer-reviewed making the test unsuitable as a specific diagnostic tool to identify the SARS-CoV-2 virus.
We find severe conflicts of interest for at least four authors, in addition to the fact that two of the authors of the Corman-Drosten paper (Christian Drosten and Chantal Reusken) are members of the editorial board of Eurosurveillance. A conflict of interest was added on July 29 2020 (Olfert Landt is CEO of TIB-Molbiol; Marco Kaiser is senior researcher at GenExpress and serves as scientific advisor for TIB-Molbiol), that was not declared in the original version (and still is missing in the PubMed version); TIB-Molbiol is the company which was “the first” to produce PCR kits (Light Mix) based on the protocol published in the Corman-Drosten manuscript, and according to their own words, they distributed these PCR-test kits before the publication was even submitted [20]; further, Victor Corman & Christian Drosten failed to mention their second affiliation: the commercial test laboratory “Labor Berlin”. Both are responsible for the virus diagnostics there [21] and the company operates in the realm of real time PCR-testing.
In light of our re-examination of the test protocol to identify SARS-CoV-2 described in the Corman-Drosten paper we have identified concerning errors and inherent fallacies which render the SARS-CoV-2 PCR test useless.
https://web.archive.org/web/20220923083309/https://cormandrostenreview.com/report/
6
The RT-PCR process is NOT a test that can diagnose illness or contagiousness. Its was never intended to be a diagnostic tool. The PCR process merely creates copies of genetic material found in a sample. The detection of certain molecules via the RT-PCR process does NOT provide evidence of disease or contagiousness. The presence of nucleic acids alone should not be used to infer disease, infection, viral shedding or potential contagiousness.
United Kingdom
A single Ct value in the absence of clinical context cannot be relied upon for decision making about a person’s infectivity.
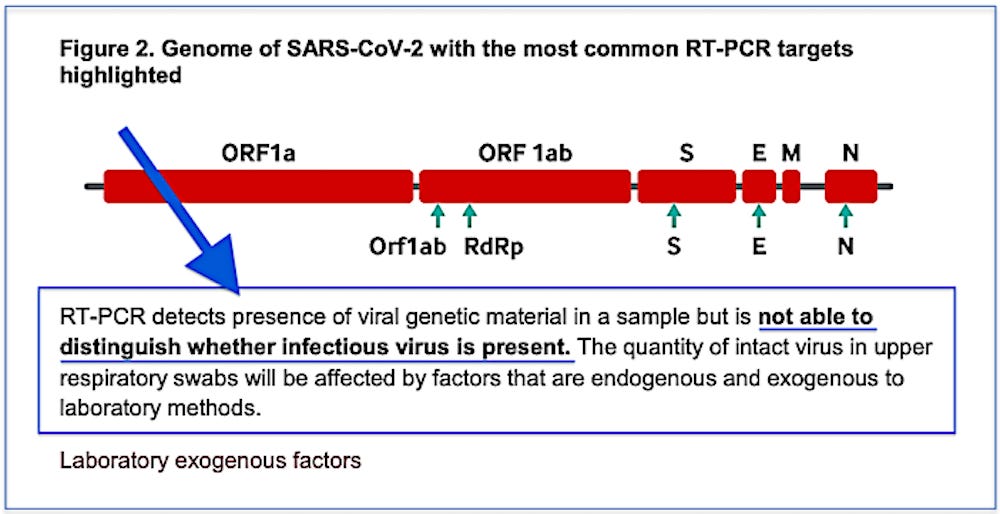
RT-PCR detects presence of viral genetic material in a sample but is not able to distinguish whether infectious virus is present.
Canada
A person is deemed infectious if they shed virus particles that are intact and able to go on to infect others. PCR tests cannot distinguish viral genomic material coming from intact viral particles in persons who are infectious or viral particle fragments that are present in individuals who have recovered.
We do not know how much virus is actually required to cause an infection in someone and there are other important factors that may influence infectiousness, including the health of the person exposed and the type of exposure that has happened.
The CDC
Detection of viral nucleic acid is not equivalent to isolating a virus.
The CDC
A positive result indicates detection of influenza viral RNA or nucleic acids in the respiratory specimen tested, confirming influenza virus infection, but does not necessarily mean infectious virus is present or that the patient is contagious.
https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
New Zealand
A positive test cannot tell us:
if the person is currently infectious
how ill the person is likely to become.
Singapore
It is important to note that viral RNA detection by PCR does not equate to infectiousness or viable virus. https://www.ncid.sg/Documents/Period%20of%20Infectivity%20Position%20Statementv2.pdf
Sweden
Comparisons of results with PCR detection of viruses and virus culture shows that PCR cannot be used to determine whether an individual remains contagious or not because PCR also detects RNA from non-infectious viruses. Sampling by PCR to determine infectiousness should therefore be avoided.
https://kommun.falkenberg.se/images/sv/files/vagledning-om-smittsamhetsbedomning-vid-covid-19%C3%85H.pdf (pages 6-7)
Nature Reviews Microbiology
Although detection of viral RNA in respiratory specimens by RT-PCR is highly sensitive and specific, it does not distinguish between replication-competent virus and residual RNA.
RT-PCR cannot directly determine infectiousness owing to its inability to differentiate between replication-competent (infectious) virus and residual (non-infectious) viral RNA.
Unfortunately, no point-of-care diagnostic test currently exists to determine infectious SARS-CoV-2 in a patient sample.
To date, no diagnostic tests exist that reliably determine the presence of infectious virus.
The Lancet
Although the use of sensitive PCR methods offers value from a diagnostic viewpoint, caution is required when applying such data to assess the duration of viral shedding and infection potential because PCR does not distinguish between infectious virus and non-infectious nucleic acid.
The presence of nucleic acid alone cannot be used to define viral shedding or infection potential.
For many viral diseases (SARS-CoV, Middle East respiratory syndrome coronavirus, influenza virus, Ebola virus, and Zika virus) it is well known that viral RNA can be detected long after the disappearance of infectious virus.
The immune system can neutralise viruses by lysing their envelope or aggregating virus particles; these processes prevent subsequent infection but do not eliminate nucleic acid, which degrades slowly over time.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30868-0/fulltext
Clinical Infectious Diseases
Complete live viruses are necessary for transmission, not the fragments identified by PCR.
Clinical Infectious Diseases
A positive RT-qPCR result may not necessarily mean the person is still infectious or that he or she still has any meaningful disease.
The RNA could be from nonviable or killed virus. Live virus is often isolable only during the first week of symptoms but not after day 8, even with positive RT-qPCR tests.
Cleveland Clinic
The test can continue to detect fragments of SARS-CoV-2 virus even after you’ve recovered from COVID-19 and are no longer contagious. So you may continue to test positive if you've had COVID-19 in the distant past, even though you can’t spread the SARS-CoV-2 virus to others.
https://my.clevelandclinic.org/health/diagnostics/21462-covid-19-and-pcr-testing
7
By design, the initial steps in the RT-PCR process destroy the source material so that the RT-PCR process CANNOT possibly measure intact viruses. A so-called “positive” result does not even ensure the presence of individual virions.
Sample preparation excludes the detection of replication‐capable viruses.
A PCR test - even if performed correctly - cannot provide any information on whether a person is infected with an active pathogen or not.
This is technically impossible, because the test procedure includes a complete destruction of the biological material and separation of nucleic acids from all other material, thus destroying any structure necessary for biological function like replication and infection.
This is because the test cannot distinguish between "dead" matter*, such as a completely harmless genome fragment as a remnant of the body's own immune system's fight against a cold or flu (such genome fragments can still be found many months after the immune system has "dealt with" the problem), and "living" matter, i.e. a "fresh", reproducible virus.
A crucial step in this process is the complete denaturation of all biological material and separation of the main components protein, lipids and nucleic acids in order to finally have the RNA available as a starting base for RT‐qPCR.
The original protocol of Chomszynski and Sacci from 1987 is still a component of almost all protocols for the purification of biological material for RNA isolation, whether prepared in the laboratory or in purchased "extraction kits." Components of the original extraction solution are phenol/chloroform and isoamyl alcohol, and in various modified commercial solutions [are] similarly acting but less toxic substances.
All have in common that they completely destroy any living or reproducible biological structure.
In the laboratory process of preparing a smear sample, which is mandatory preceeding the RT-qPCR, any biological material, be it a vital cell, a replicable virus or even just cell debris and gene residues, is denatured in such a way, that it is no longer possible to say whether the material originates from an intact or even replication competent organism or from samples that have already been damaged or destroyed.
Due to this extraction and preparation process, a positive RT-qPCR that detects genome fragments cannot be used to infer the presence of replication-capable viruses in the smear sample.
Thus, even if the PCR, including all preparatory steps (PCR design and establishment, sample collection, preparation and PCR performance), is carried out "correctly", and the test is positive, i.e.: detects a genome sequence which may also exist in one or even the specific "Corona" virus (SARS-CoV-2), this technique can under no circumstances prove that the person who tested positive could be infected with a replicating SARS-CoV-2 and consequently infectious = dangerous for other persons.
Expert opinion by Prof. Dr. rer. biol. hum. Ulrike Kämmerer
Expert Opinion Ulrike Kmmerer Phd
1.36MB ∙ PDF file
8
The potential for false positives from use of the RT-PCR process is enormous. Even if the specificity of a “test” is 99%, if the prevalence of infection in the community is 1/100, then the “test” will return a false positive result 50% of the time.
United Kingdom
What causes false positives?
Cross reactions with other genetic material. Other sources of DNA or RNA may have cross reactive genetic material that can be amplified by the RT-PCR test. False positives were observed unexpectedly in norovirus assays in patients with enterocolitis, due to unusually high levels of human DNA in samples.
Contamination during sampling. This may happen if the swab head accidently contacts, or is placed on a contaminated surface (for example, latex gloves, hospital surface).
Contamination during swab extraction.Viral RNA is extracted from swabs in solution; accidental aerosolization of liquid can cause cross contamination between samples.
Contamination with PCR amplicon. The PCR amplification process generates millions of copies of the DNA target (amplicon) that can cause false positives in subsequent PCR reactions. If a testing lab is accidently contaminated with amplicon it can lead to sporadic false positives.
Contamination of PCR laboratory consumables. Contamination can spread from a post-PCR lab into a pre-PCR lab by transfer of equipment, chemicals, people or aerosol. Even experienced national labs can be affected. In early-March 2020, COVID-19 RT-PCR assays produced by the CDC were withdrawn after many showed false positives due to contaminated reagents.
World Health Organization
WHO reminds [In Vitro Diagnostic Medical Device] IVD users that disease prevalence alters the predictive value of test results; as disease prevalence decreases, the risk of false positive increases (2). This means that the probability that a person who has a positive result (SARS-CoV-2 detected) is truly infected with SARS-CoV-2 decreases as prevalence decreases, irrespective of the claimed specificity.
https://www.who.int/news/item/20-01-2021-who-information-notice-for-ivd-users-2020-05
FDA
Positive and negative predictive values are highly dependent on prevalence. False-negative test results are more likely when prevalence of disease is high. False-positive test results are more likely when prevalence is moderate to low.
FDA
Remember that positive predictive value (PPV) varies with disease prevalence when interpreting results from diagnostic tests. PPV is the percent of positive test results that are true positives. As disease prevalence decreases, the percent of test results that are false positives increase.
For example, a test with 98% specificity would have a PPV of just over 80% in a population with 10% prevalence, meaning 20 out of 100 positive results would be false positives.
The same test would only have a PPV of approximately 30% in a population with 1% prevalence, meaning 70 out of 100 positive results would be false positives. This means that, in a population with 1% prevalence, only 30% of individuals with positive test results actually have the disease.
At 0.1% prevalence, the PPV would only be 4%, meaning that 96 out of 100 positive results would be false positives.
Health care providers should take the local prevalence into consideration when interpreting diagnostic test results.
The Lancet
The widespread use of PCR in clinical settings has been hampered largely by background contamination from exogenous sources of DNA. In most pathogen-specific assays, the predominant source of contamination is derived from “carryover” products from earlier PCR reactions, which can be harboured and transmitted through PCR reagents, tubes, pipettes, and laboratory surfaces. Coupled with the robust amplification power of PCR, even very minor amounts of carry-over contamination may serve as substrates for amplification and lead to false-positive results.
Journal of Infection
In light of our findings that more than half of individuals with positive PCR test results are unlikely to have been infectious, RT-PCR test positivity should not be taken as an accurate measure of infectious SARS-CoV-2 incidence. Our results confirm the findings of others that the routine use of “positive” RT-PCR test results as the gold standard for assessing and controlling infectiousness fails to reflect the fact “that 50-75% of the time an individual is PCR positive, they are likely to be post-infectious
There is no international standardization across laboratories, rendering problematic the interpretation of RT-PCR tests when used as a tool for mass screening.
https://www.journalofinfection.com/article/S0163-4453(21)00265-6/fulltext
The Royal College of Pathologists of Australasia
Timely identification of true false positive SARS-CoV-2 NAT results is important as unrecognised false positive results can lead to unnecessary quarantining and contact tracing, delays in the recognition and treatment of the true illness, significant patient anxiety and concern, potential exposure to nosocomial infection from other patients with confirmed COVID-19, wastage of personal protective equipment, and inaccurate statistics regarding local prevalence of infection.
https://www.pathologyjournal.rcpa.edu.au/article/S0031-3025(20)30936-3/fulltext
Clinical Medicine
Several potential significant implications for the single-gene low-level false positive results were recognised. Patients on the transplant waiting list were removed from the list for 2 weeks. Some of the patients screened pre-operatively had their surgery delayed. Patients screened pre-discharge were kept in hospital, unnecessarily in many cases.
Implications of false positive results
unnecessary treatment and investigation
missing or delayed surgery
unnecessary isolation and contact tracing with subsequent negative impact on workforce and resources
a risk of subsequent increased exposure if the individual changes their behaviour as a result of believing that they have been infected
the individual being placed with other inpatients with COVID-19 and consequently exposed to the virus.
A major risk of a false positive result occurs when the individual is cohorted with other patients suffering from COVID-19 and is consequently exposed to the virus.
Journal of Occupational and Environmental Medicine
The performance of these tests when deployed depends not only on their clinical sensitivities and specificities, but also the prevalence of SARS-CoV-2 infections in the setting in which the test is being used. If we consider a test that conforms to the FDA's recommendations for performance in a… screening setting (95% sensitivity, 98% specificity).
For the screening scenario, 100 of the 10,000 individuals are infected and 9900 are not. The test will detect 95 of the infected persons and five will be falsely negative. For those who are not infected, 9702 will be correctly diagnosed and 198 will be false positives. The PPV is 95/95 + 198 or 32.4%. In this case, 2/3 of the positive results are false positives. For a prevalence of 0.1%, the PPV drops to 4.5%.
9
There is ample evidence that any claims of a “positive” result obtained by running the RT-PCR process through more than 24 cycles are actually false positives.
World Health Organization
Careful interpretation of weak positive NAAT results is needed, as some of the assays have shown to produce false signals at high Ct values.
https://iris.who.int/bitstream/handle/10665/334254/WHO-2019-nCoV-laboratory-2020.6-eng.pdf
Canada
A frequent question is whether Ct values can help determine whether an individual is infectious or not. It is not possible to directly translate a Ct value into degree or duration of infectiousness.
Clinical Infectious Diseases
At Ct = 35, the value we used to report a positive result for PCR, <3% of cultures are positive.
Clinical Infectious Diseases
Many qPCR assays involve a Ct cutoff of 40 to consider the test positive, allowing detection of very few starting RNA molecules.
However, reporting as a binary positive or negative result removes useful information that could inform clinical decision making.
Following complete resolution of symptoms, people can have prolonged positive SARS-CoV-2 RT-PCR test results, potentially for weeks, as Xiao et al report. At these late time points, the Ct value is often very high, representing the presence of very low copies of viral RNA [5–8]. In these cases, where viral RNA copies in the sample may be fewer than 100, results are reported to the clinician simply as positive. This leaves the clinician with little choice but to interpret the results no differently than for a sample from someone who is floridly positive and where RNA copies routinely reach 100 million or more.
A positive RT-qPCR result may not necessarily mean the person is still infectious or that he or she still has any meaningful disease.
First, the RNA could be from nonviable or killed virus. Live virus is often isolable only during the first week of symptoms but not after day 8, even with positive RT-qPCR tests [9].
Second, there may need to be a minimum amount of viable virus for onward transmission. For infection control purposes, the utility of the assay is greatest when identifying people who are floridly positive and at risk of further transmission. Particularly when testing in the absence of symptoms for COVID-19, we believe that reporting the Ct value or range could help to better inform clinical decisions.
Clinical Infectious Diseases
Above a CT value of 24, the amount of detectable viral genetic material is so low that the positive test could no longer be interpreted in terms of an active infection.
European Journal of Clinical Microbiology & Infectious Diseases
We can deduce that with our system, patients with Ct values equal or above 34 do not excrete infectious viral particles.
https://link.springer.com/article/10.1007/s10096-020-03913-9
The New England Journal of Medicine
Viral culture was positive only in samples with a cycle-threshold value of 28.4 or less.
10
There is no proof that the nucleic acid sequences that are utilized in any of the various RT-PCR “tests” identify the presence of a viable pathogen that causes COVID-19. An unknown number of other pathogens that are NOT being tested for may also be present. Even if the RT-PCR process identifies the existence of genetic remnants of SARS-CoV-2, it does not rule out the possibility that something else may be the actual cause of disease.
World Health Organization
Co-infections of SARS-CoV-2 with other pathogens have been reported, thus a positive test for another pathogen does not rule out COVID-19 and vice versa.
https://iris.who.int/bitstream/handle/10665/334254/WHO-2019-nCoV-laboratory-2020.6-eng.pdf
FDA
Detection of viral RNA may not indicate the presence of infectious virus or that 2019-nCoV is the causative agent for clinical symptoms.
This test cannot rule out diseases caused by other bacterial or viral pathogens.
FDA
Clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule out bacterial infection or co-infection with other viruses.
Eurosurveillance
In total, we have tested to date (as at 19 February 2020) 4,084 respiratory samples by PCR and all the tests have been negative for SARS-CoV-2.
These tests were carried out on the samples of 32 suspected SARS-CoV-2 cases, 337 people repatriated at the beginning of February 2020 from China tested twice, 164 patients who died in public hospitals in Marseille between 2014 and 2019 of whom at least one respiratory sample had been sent to our laboratory, and they also included 3,214 respiratory samples sent since January 2020 to our laboratory to search for a viral aetiology.
In striking contrast, we have tested 5,080 respiratory samples for various suspected respiratory viral infections since 1 January 2020 and identified in 3,380 cases respiratory viruses. In decreasing order of frequency, they were: influenza A virus (n = 794), influenza B virus (n = 588), rhinovirus (n = 567), respiratory syncytial virus (n = 361), adenovirus (n = 226), metapneumovirus (n = 192), enterovirus (n = 171), bocavirus (n = 83), parainfluenza virus (n = 24), and parechovirus (n = 8). Among the diagnosed viruses, there were also 373 common human coronaviruses (HCoV), including 205 HCoV-HKU1, 94 HCoV-NL63, 46 HCoV-OC43, and 28 HCoV-229E [5].
Thus, it is surprising to see that all the attention focused on a virus whose mortality ultimately appears to be of the same order of magnitude as that of common coronaviruses or other respiratory viruses such as influenza or respiratory syncytial virus, while the four common HCoV diagnosed go unnoticed although their incidence is high.
https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.8.2000171
Clinical Chemistry and Laboratory Medicine
A total of 239 positive targets of pathogens were detected in 161 children. The highest proportion of pathogens were human respiratory syncytial virus (HRSV) (in 76 patients [31.80%]) and influenza A virus (in 72 patients [30.13%]).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, was detected in two patients and accounted for 0.84%.
SARS-COV-2, HRSV and human metapneumovirus (HMPV) were found in the bronchoalveolar lavage fluid of patient 1, and SARS-COV-2, MP and HMPV were found in the bronchoalveolar lavage fluid of patient 2.
https://www.degruyter.com/document/doi/10.1515/cclm-2020-0434/html
The Lancet [Comment]
In our view, current PCR testing is therefore not the appropriate gold standard for evaluating a SARS-CoV-2 public health test.
The short window of transmissibility contrasts with a median 22–33 days of PCR positivity (longer with severe infections and somewhat shorter among asymptomatic individuals). This suggests that 50–75% of the time an individual is PCR positive, they are likely to be post-infectious.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00425-6/fulltext
International Journal of Vaccine Theory, Practice, and Research
It was revealed that a positive RT-qPCR test still harbors the risk of picking up something other than SARS-CoV-2 alone. Samples from primary care patients suspicious for SARS-CoV-2, since the patients presented themselves with clinical symptoms of a respiratory infection, after RT-qPCR, were found to be positive for other viral and bacterial pathogens and even human genomic DNA (Voogd et al., 2022). Thus, solely trusting the outcome of a positive RT-qPCR test result risks a wrong diagnosis even with optimized commercial kits.
All that being said, nevertheless, we believe there was a grossly negligent omission during the COVID-19 pandemic: regular and sufficient negative and positive controls did not exclude the co-presence of pathogen(s) other than SARS-CoV-2(except in the just-mentioned Killingley experiment) which might be causing the observed COVID-19 disease symptoms. Additionally, the symptoms used in diagnosis are so general and common in respiratory diseases that it “... may not be possible to distinguish among the viral diseases under study judging only by the clinical presentation” (Czubak et al., 2021). Among the disease agents that cannot be definitively excluded are seasonal flu viruses which have been identified as co-infective by Wuhan researchers (Yue et al., 2020) in some persons diagnosed withSARS-CoV-2 infection. Given that there are no “virus-type-specific” therapies that distinguish all the types of respiratory viruses, molecular diagnostics hardly have anything more than mere academic value. The critical information guiding the choice of therapies would need to take account of co-infecting bacteria and fungi possibly accompanying any respiratory viruses and for which a specific therapy could have helped or even saved the lives of many victims —such as those “COVID-19 patients” who died with non-detected aspergillus and might have survived on anti-fungal therapy (Evert et al., 2021).
We assert that testing asymptomatic people is useless.
The article above is the condensed version of the evidence against the use of the RT-PCR process as a diagnostic “test.”
CLICK HERE to access additional information regarding the RT-PCR Fraud.
James Roguski
310-619-3055
JamesRoguski.substack.com/archive
All content is free to all readers.
All support is deeply appreciated.


















No comments:
Post a Comment