New paper in peer review confirms Covid-19 vaccine batch variability in both Denmark and Sweden
I would like to bring your attention to this paper, newly published in peer review. I know the authors, and their work is top notch. This study is the continuation of the work by Vibeke Manniche and Max Schmeling on batch variability data:
The new paper looks at the same data in Sweden and makes comparison to the Danish data.
“Reports of Batch-Dependent Suspected Adverse Events of the BNT162b2 mRNA COVID-19 Vaccine: Comparison of Results from Denmark and Sweden”.
Authors:
Vibeke Manniche, Max Schmeling, Jonathan D. Gilthorpe, Peter Riis Hansen
Authors’ affiliations:
LIVA, Rosenvængets Allé 6A, DK-2100 Copenhagen, Denmark
Innometric, Bavnehøjvej 5, Hellum, DK-9520 Skørping, Denmark
Department of Medical and Translational Biology, Umeå University, 901 87 Umeå, Sweden
Department of Cardiology, Copenhagen University Hospital—Herlev and Gentofte, Gentofte Hospitalsvej 1, DK-2900 Hellerup, Denmark
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark
Citation:
Medicina 2024, 60(8), 1343; https://doi.org/10.3390/medicina60081343
Abstract:
Background and Objective: An unexpected batch-dependent safety signal for the BNT162b2 mRNA COVID-19 vaccine was recently identified in a nationwide study from Denmark, but the generalizability of this finding is unknown. Therefore, we compared batch-dependent rates of suspected adverse events (SAEs) reported to national authorities in Denmark and Sweden. Materials and Methods: SAE and vaccine batch data were received from national authorities in Denmark and Sweden, and analyses of heterogeneity in the relationship between numbers of vaccine doses and SAEs per batch were performed, along with comparison of SAE rates and severities for batches that were shared between the two countries. Results: Significant batch-dependent heterogeneity was found in the number of SAEs per 1000 doses for both countries, with batches associated with high SAE rates detected in the early phase of the vaccination campaign and positive correlations observed between the two countries for the severity of SAEs from vaccine batches that they shared. Mild SAEs predominated in the batches used in the early part of the vaccination roll-out, where markedly higher SAE rates per 1000 doses in Denmark for the batches that were shared between the two countries suggested that a large proportion of these SAEs were under-reported in Sweden. Conclusions: The batch-dependent safety signal observed in Denmark and now confirmed in Sweden suggests that early commercial batches of BNT162b2 may have differed from those used later on, and these preliminary and hypothesis-generating results warrant further study.
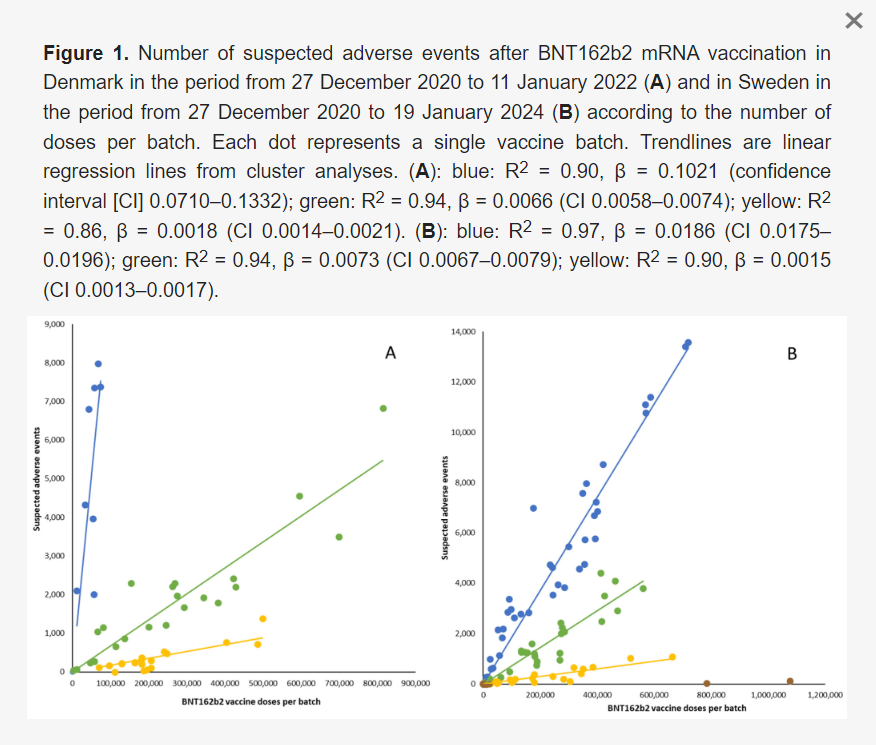
Results: Batches administered in Sweden showed the same non-random, high variability pattern as previously demonstrated for Denmark. However, the toxicity slopes of the 3 groupings were different, even though many batches were shared between the 2 countries.
Discussion:
The present study extends our previous report on significant heterogeneity in BNT162b2 batch-dependent SAE rates for the BNT162b2 vaccine in Denmark by demonstrating a similar batch-dependent safety signal in nationwide data from Sweden. Both countries had high-SAE-rate batches in the early phase of vaccine roll-out, followed by declining rates of batch-dependent SAE rates over time, and summary demographic data did not provide any obvious explanation for these observations. Also, a positive correlation was observed between the two countries regarding the severities of SAEs from the batches that they shared. Notably, however, Danish batch-dependent SAE rates per 1000 doses exhibited somewhat more pronounced heterogeneity than the Swedish data, especially in the early phase of the vaccination campaign and with regard to mild SAEs, where markedly lower SAE rates were reported in Sweden for batches shared between the two countries.[…]
A commercial vaccine product should be identical in all batches including those shared between countries, and it is surmised that vaccinated individuals in Denmark and Sweden objectively encountered the same rates of SAEs and had similar means and opportunity to report SAEs. Therefore, our data suggest that mild SAEs were markedly under-reported in Sweden during the early phase of vaccine roll-out. The factors that modulate the spontaneous reporting of SAEs on a population scale are multifactorial, but it is notable that the governmental response to the COVID-19 pandemic differed considerably in Denmark and Sweden, with a high hierarchical command and control governance in Denmark (including societal lockdown and selective engagement with healthcare expertise) and a network-based approach with extensive regional and local autonomy in Sweden. For example, in June 2021, i.e., during the period of the administration of the ‘green’ moderate SAE rate batches, the DKMA issued a request for the public not to report simple and transient SAEs, whilst to our knowledge, similar regulatory pleas were not issued in Sweden. Altogether, the differing control governance arrangements in Denmark and Sweden might, therefore, have suggested that, contrary to our findings, the under-reporting of SAEs in the early phase of the vaccination campaign would be most likely in Denmark compared with Sweden. However, we also observed that during the study period, a considerably higher percentage of SAE reports (40 vs. 15%) were from healthcare workers rather than private individuals in Denmark compared to Sweden. Interestingly, periodic yearly reports from the SMPA summarizing SAE reports for all marketed pharmaceutical products in Sweden have shown a distinct shift in ratios of SAE reports from healthcare workers/the public in 2021, with ratios of 72/28% in 2020 and 53/47% in 2021, respectively, with this shift mainly determined by changes in reporting ratios for the COVID-19 vaccines. Whether healthcare workers in Sweden were less inclined to report SAEs from the BNT162b2 vaccine in Sweden requires further study, including to determine the potential role of the perceived risk that such reports may be linked to repressive measures from governmental and regional health authorities. Notably, the importance of political observance for the reporting of SAEs related to COVID-19 vaccines was recently suggested in a study from the US Vaccine Adverse Events Reporting System (VAERS), a passive surveillance system like the reporting systems managed by the DKMA and SMPA, which showed that the more likely it was that US states voted Republican, the more likely these states were to report vaccine-related SAEs. In any case, the under-reporting of data that best represent objective rates of SAEs may have important public health consequences, e.g., by impeding long-term follow-up of subjects with SAEs and reducing the likelihood of backtracking later clinical events to SAEs. Also, it seems plausible that if short- and medium-term SAEs are under-reported, then long-term SAEs are even less likely to be reported.
The current validation by Swedish data of the batch-dependent safety signal reported from Denmark adds weight to the hypothesis that early commercial BNT162b2 vaccine batches may have differed from the latter batches and that batch-level product quality surveillance and pharmacovigilance may have been suboptimal during the BNT162b2 vaccine roll-out.
My own interpretation of this data is far less charitable than the authors of the paper present. It’s not a matter of improving quality control of vaccine production. The quality is unimprovable, because covid vaccines, like all vaccines are intentional poison, so improving quality just makes it a more pure, more consistent poison.
While I no longer focus on the CDC VAERS database, I did a lot of data analyses in 2021 and 2022 identifying manufacturing inconsistency and lack of compliance with the current Good Manufacturing Practices (cGMP) observable immediately post the mRNA/DNA injections rollout. I used VAERS database to study variability of serious adverse events (life-threatening, disability, miscarriages, hospitalizations, ER visits or doctor visits) and deaths based on lot numbers. Lot or batch numbers represent vials produced and filled in a single production run (batch) and are printed on vials, and are supposed to be recorded on the vax cards and in vax injury reports. People do not always have the vax card when filling VAERS reports, so often the lot numbers are missing. In many cases they appear to be mistyped. I say “appear” because many investigators, including myself detected algorithmic manipulation of VAERS data and it is likely that CDC’s contractors change the lot numbers on many reports to obfuscate the detection of safety signals.
In 2021 I met Craig Paardekooper, a pharmacy student from the UK, who had to leave the university due to the vax mandates. We collaborated with a few other database/software/data analysts under the name “Team Enigma”.
We now know mRNA shots are weaponized tech in a vial, but back in 2021 we were still naive and thinking this was supposed to be a pharmaceutical product. Craig put up a website How Bad Is Your Batch (www.howbad.info) to present the data to the public. It has since grown into a very successful educational and data resource. The website presents a huge amount of collective research and a record of the worldwide genocide unfolding.
We have shown that the batches released in the US between December 2020 and April 2021 had markedly higher apparent toxicity per dose, however, huge variability batch to batch remained, even after overall levels of toxicity decreased later in 2021-2023. This is not mentioned by the authors of the above study, but my own conclusion was that the usage of vaccines declined very significantly after April 2021, while the batches became larger and larger, drowning the toxicity signal per dose in unused product that sat on the shelves. This is explained in this article:
The tight association of adverse events with the usage of vials per lot only proves one more time that the vaccines are toxic and deadly.
An ability to manufacture the product to cGMP standards determines whether a pharmaceutical product can exist in the first place. That comes before clinical trials, before safety and efficacy can be even assessed. If you cannot demonstrate that you are making the XYZ formulation every time, in every pill, vial, capsule, in millions and billions of them, in every batch - you cannot even begin to assess whether XYZ is safe or efficacious for anything!
As I and Katherine Watt have discussed many times, vaccines are not regulated as pharmaceuticals, in fact, they have always been fake-regulated and used as a tool of systematic poisoning of the population. The mRNA vax batches are the most toxic vaccines ever produced.
May help more people understand that vaccines, from the batch and lot level at the factories, through the vial and dose level when administered to a person, are intrinsically heterogeneous, unstable and toxic.
There is no safe dose of vaccine material.
There never will be.
The vax batches are not manufactured to cGMP/EU Pharm standards and are not the same, and there is huge variability in death and injury even in the same batches when they are split between countries, like the authors show here between Sweden and Denmark. I and my collaborators found the same phenomenon occurring between different US states.
Art for today: Flats, watercolor, 9x12 in.








No comments:
Post a Comment