Long COVID is an Autoimmune Disease: Injecting Mice with IgG from Long COVID Patients Induces Symptomology
Revisiting One of My Original Spike Protein Hypotheses in A New Light: The Molecular Mimicry of the Spike Protein Will Induce Autoimmune Disease
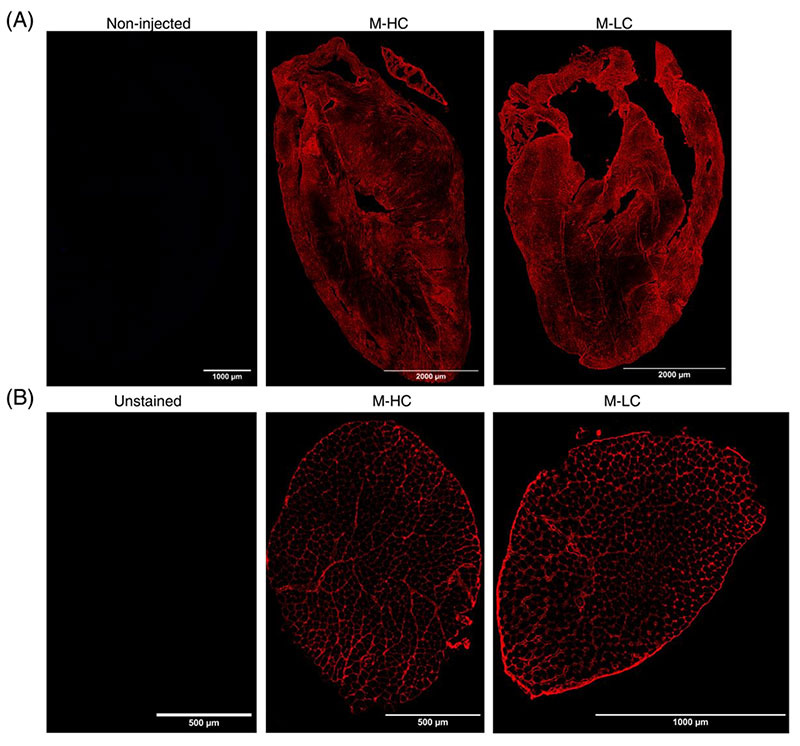
Injected human IgG (hIgG) of Long COVID (LC) patients and healthy donors (HC) localizes in murine heart and skeletal muscles. (A) Representative staining of hIgG (red) in the heart of M-HC (middle panel) and M-LC (right panel). As a negative control, hearts of non-injected mice were included and stained for the presence of hIgG (left panel). (B) Representative staining of hIgG in skeletal muscle of M-HC (middle panel) and M-LC (right panel). As a negative control, unstained skeletal muscle was taken along (left panel).
On November 23, 2021 I wrote that the molecular mimicry of the Spike Protein was inducing autoimmune disease which itself mimics Graft vs Host Disease.
PHASE 2 – DONOR T CELL ACTIVATION
This is where things get tricky and interesting. In Phase 2 of GVHD, donor T cell activation, proliferation, differentiation and migration in response to primed APCs occurs during the second phase of acute GVHD. The T cell receptors (TCR) of donor T cells recognize alloantigens on both host and donor type APCs that are present in secondary lymphoid organs. During direct presentation, donor T cells recognize either the peptide bound to host MHC molecules, or the foreign MHC molecules themselves.
However, in COVID-19 it is YOUR OWN T cells which are activating, proliferating, differentiating and migrating in response to the primed APCs. The homologies in the Spike Protein are the “alloantigens” – except, they also happen to be yours.
PHASE 3 – CELLULAR AND INFLAMMATORY EFFECTOR PHASE
A complex cascade of cellular and inflammatory mediators occurs during the effector phase of acute GVHD and COVID-19. These mediators synergize to amplify local tissue injury and damage target tissues. As such, the effector phase of GVHD and COVID-19 involves aspects of both the innate and adaptive immune response as well as interactions with the proinflammatory cells and cytokines generated during phase 1 and 2.
DESTRUCTION OF TARGET TISSUES. Heart. Lung. Gastrointestinal. Brain. Etc. Followed by – FIBROSIS.
This is Severe COVID. This is Long COVID. This is MIS-C, MIS-A and MIS-V.
COVID-19 AND GRAFT VS HOST DISEASE
https://wmcresearch.org/covid-19-and-graft-vs-host-disease/
A preprint was published May 30th that details how Long COVID is, at least in part, an autoimmune disease. But first, let me explain a mechanism that can enhance the autoimmune inducing molecular mimicry of the Spike Protein. This example comes from studying vaccines.
Nonetheless, attention is rarely drawn to a significant environmental factor applied regularly with the sole purpose of impairing immune tolerance—Adjuvants.
The term adjuvant refers to a variety of compounds used to stimulate immune response. In fact, research of immune responses is dependent on these compounds since inducing a significant ‘researchable’ immune response to pathogenic elements is possible almost solely when injecting these element along with an adjuvant.12
Kanduc11 suggested the need for adjuvants stems from the inherent tolerance of the human immune system to many pathogenic motifs, which are repeatedly shared with the human proteome. Therefore, she offered that upon exposure of the immune system to these shared motifs, while impairing immune tolerance (by adding an adjuvant), a reasonable outcome may be the development of crossreactivity and autoimmunity.11
This application of the molecular mimicry theory may serve to explain potential development of autoimmune phenomena post vaccination.
Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6078966/
Now, what makes this very interesting and unique is that the Spike Protein IS ITS OWN ADJUVANT! It, of its own accord, “revs up” the immune system, like an adjuvant.
Hence, the mRNA vaccine “theory” neglects the possibility that any cell producing the Spike protein and displaying it on its membrane (associated or not with MHC-I) will be attacked and destroyed by CD8+T cells. The severity of the consequences for the host following the vaccination will depend on the type and number of cells affected and the tissue where the reaction occurs. For example, myocarditis is considered an adverse reaction to mRNA vaccination [85,86]. The facts that this event is more frequent after the second dose and it occurs a few days after the inoculation [27], suggest an immune-mediated mechanism analogous to an auto-immune reaction. To conclude, the Spike protein acts in a peculiar way, not simply as an immunogen, but as a disease-causing agent.
Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9953067/
So, all of this leads us to the preprint. What was discovered was that if you take antibodies from individuals with Long COVID and inject them into mice, you induce Long COVID symptoms. This, of course, sounds logical, but that’s not the point. The point is that the antibodies are attacking self.
Here, we demonstrated that the transfer of IgG from Long COVID patients induced behavioral symptoms in mice, indicating the causal role of IgG in Long COVID pathophysiology. Importantly, the pattern and nature of behavioral changes was different depending on which subgroup of LOC patients the IgG were derived. Mice injected with IgG from LC-1, the patients with elevated plasma levels of neurodegenerative proteins (GFAP and NFL), exhibited the significant increase in mechanical and thermal sensitivity from day 3 to 15 post-intraperitoneal IgG injection. This aligns with the association between astrogliosis, nerve damage and chronic pain [53]. Additionally, MMP1, the top elevated plasma marker in LC-1 patients (Supp. Fig. 3A), can promote sustained pain by inducing neurostructural changes and PAR1 signaling [54, 55]. Mice that received IgG from LC-3 developed a more rapid onset of hypersensitivity post-injection. LC-3 patients’ plasma had higher levels of leukocyte activation proteins, with one of the top upregulated proteins being EIF5A (Supp. Fig. 3C). Intriguingly, inhibition of EIF5A reduces firing in human induced pluripotent stem cells (iPSC)derived neurons and prevents mechanical hypersensitivity in a model of hyperalgesic priming [56]. MThe copyright holder for this preprint bioRxiv preprint doi: https://doi.org/10.1101/2024.05.30.596590 ; this version posted May 31, 2024. (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC 4.0 International license . LC2 mice did not develop significant hypersensitivity to mechanical and heat stimulation. Interestingly, compared to LC-1 and LC-3 patients, the corresponding patients (LC-2) exhibited lower plasma levels of neural proteins, particularly involved in the regulation of neurotransmitter transport and secretion (Fig. 3B). In contrast, LC-2 patients’ plasma was enriched in proteins in skeletal and cardiac muscle pathways. For instance, TTN, one of the top enriched protein in these patients (Fig. 3B), is responsible for the passive elasticity of muscle, and its mutation has been associated with muscle disorders and cardiomyopathies [57]. Mice receiving LC-2 IgG exhibited a significant reduction in distance traveled but without clear motor coordination defects and or grip strength. As motor coordination defects are often associated with neurological pathology, the plasma protein signatures are in line with the observed motor changes in mice that received IgG from LC-2. Moreover, we stratified LC-2 based on increased IFN-β, and high levels of type-I IFN decrease healthy muscle stem cell proliferation [58] and induces contractile weakness, particularly by IFN-β [59]. The potential pathological association between plasma proteome signatures and IgG-induced murine behavioral outcomes underscores the pivotal role of IgG in Long COVID's heterogeneous pathogenesis. Further investigation is warranted to elucidate if IgGs from individual patients induce varied pathological effects.
Transfer of IgG from Long COVID patients induces symptomology in mice
https://www.biorxiv.org/content/10.1101/2024.05.30.596590v1.full.pdf
I believe this provides strong, convincing evidence that Long COVID is, indeed, an Autoimmune Disease almost certainly induced by the Spike Protein. I will continue investigating to further understand the mechanisms at work and to find therapeutics to treat this smoldering disease.
Thank you, again and always, for your continued support, readership and dialog. As I have always maintained, and recently stated, I couldn’t do this without your support.


No comments:
Post a Comment