1/200 chance of death in context of new bird flu injection - 5 times higher than placebo according to clinical trial
Clinical Trial NCT02839330 tested the H5N1 Subunit Influenza Virus Vaccine
Correction and update:
Thank you to Maarten Fornerod for pointing this out! The product about to be launched in Finland is a different product from the one called AUDENZ . The information is still pertinent, and the product about to be issued in Finland is made by CSL Seqirus.
The other, also from Seqirus, is Incellipan(pandemic influenza vaccine (H5N1) (surface antigen, inactivated, adjuvanted, also from Seqirus, prepared in cell cultures)), is a pandemic preparedness vaccine intended for use only if a flu pandemic has been officially declared. In the event of a pandemic, once the virus strain causing the pandemic is identified, the manufacturer can include this strain in the authorized pandemic preparedness vaccine and apply for the vaccine to be authorized as a 'final' pandemic vaccine. Because the quality, safety and efficacy of the vaccine has already been assessed with other potential pandemic strains, the authorization of the final pandemic vaccine can be accelerated.
On June 26, 2024, Reuters wrote a pieceannouncing the looming threat of plans to offer preemptive bird flu injection to the people of Finland who work with animals.
It’s important that the people of the world understand something about the so-called AUDENZ™ (Influenza A (H5N1) Monovalent Vaccine, Adjuvanted), another product in clinical trials that will soon be offered to the public to fight ‘bird flu’.
Some details: It’s a 2-doser - 21 days apart. Anaphylaxis is counter-indicated and a precautionary warning pertaining to Guillain-Barré syndrome is listed in the prescribing information sheet.
Please open the document and proceed to section 6 - ADVERSE REACTIONS, subsection 6.1 under Clinical Trials Experience, on page 3 it is written:
It is possible that broad use of AUDENZ could reveal adverse reactions not observed in clinical trials.
What kind of adverse reactions are we talking about so far as per their trials? Let’s have a look.
Clinical safety data for AUDENZ in adults (18 years of age and older) have been collected from three studies: Study 1 in adults 18 through 64 years of age (NCT01776541); Study 2 in adults 65 years of age and older (NCT01766921), and Study 3, a placebo-controlled trial in adults 18 years of age and older (NCT02839330). The safety population includes 3,579subjects who received at least one dose of AUDENZ. Of these, 1,683 were adults 18 through 64 years of age and 1,896 were adults 65 years of age and older.
I am going to go right to the punchline here. No need for ‘efficacy assessment’. Go to Page 6. Read this slowly. And share this information please.
According to the outcome measure following 387 days of study as part of ‘study 3 - NCT02839330’: A Study to Evaluate Safety, Immunogenicity, and Lot-to-Lot Consistency of H5N1 Subunit Influenza Virus Vaccine in Healthy Adult Subjects ≥18 Years of Age:
“Fatal SAEs included 11 (0.5%) AUDENZ recipients and 1 (0.1%) placebo recipients.”
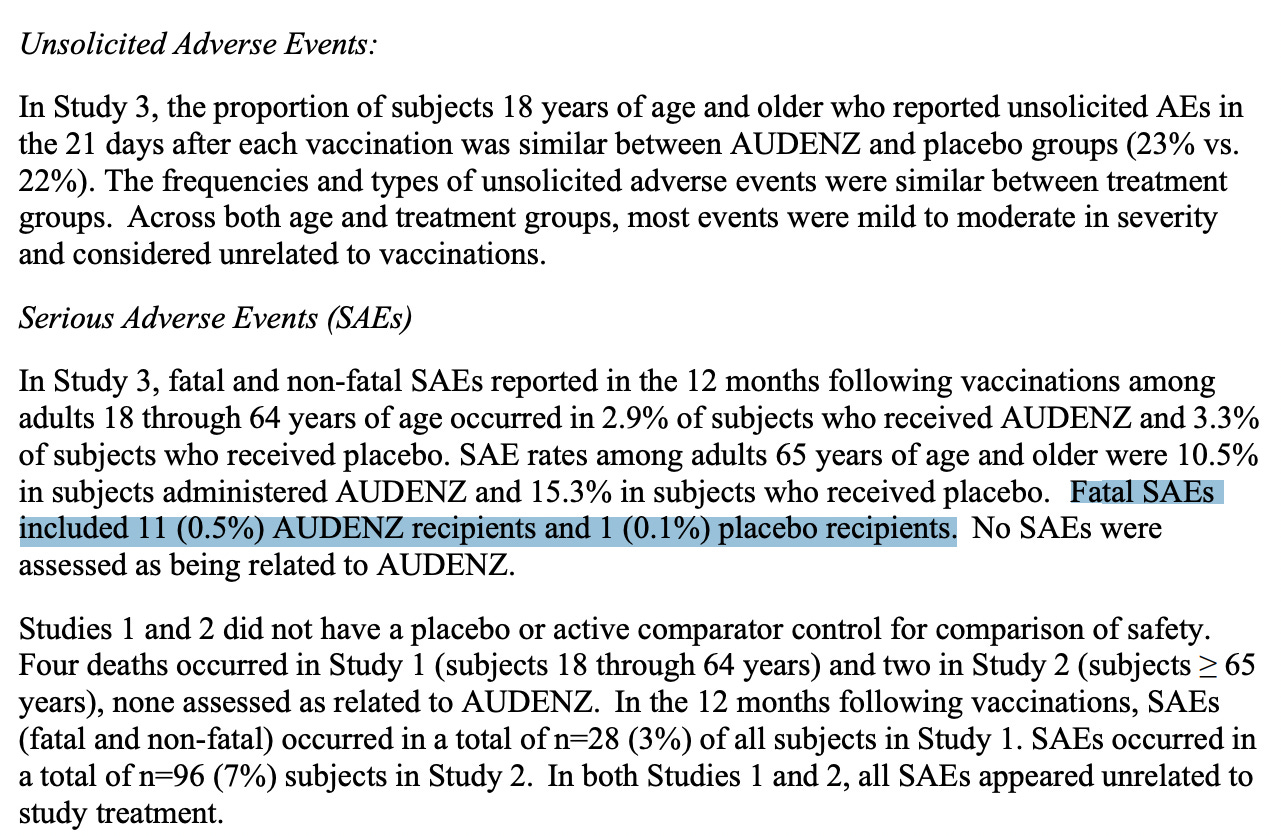
11/2,394 (0.5%) of people died on drug. That’s about 1 out of every 218 people. Died. 1/797 died on placebo. And even though the subjects were randomized in 3:1 ratio (drug:placebo), this still is not a good result. (Thanks Jonathan - this is good input. Substack rules.) It might also be important for me to point out that they went by immunogenicity according to Hemagglutination Inhibition (HI) antibody titers.
This means that of this small cohort of people, ages 18 years of age and up, that were injected with this product twice, according to their death rates, 5 times as many people died on the drug than for the placebo. Again, this means the odds of dying are ~1/200. Read that again. I thought prophylactic vaccines were meant to save you from death. I must be wrong about that.
Let’s take Finland as an example population. The population of Finland is 5,603,851. If even 5% of this population are working with animals, they are targeted for injection within a week. That means, according to their data, 1,401 animal workers will die from these shots if 280,193 are injected.
By the way, the clinical trial has a long exclusion criteria list, consists of a small cohort of people, and provides no long-term data so their rate might be underestimated.
By the way, the placebo was listed as being saline.
https://www.thepharmaletter.com/biotechnology/latest-novel-medicines-recommended-for-approval-by-ema-s-chmp
https://en.wikipedia.org/wiki/Demographics_of_Finland

No comments:
Post a Comment