Addressing allegations that DNA contamination in the mRNA shots is ‘misinformation’
Following increased public interest in the issue of excessive synthetic DNA contamination in the modified RNA (mod-RNA) Covid vaccines, Australia’s drug regulator, the Therapeutic Goods Administration (TGA) issued a statementalleging that the scientific evidence of the contamination is invalid, and that online reporting of the issue is “misinformation.”
This statement contains false and misleading claims, but does not contain any evidence to refute the contamination findings and implications. Nevertheless, the TGA’s statement has been quoted by media and politicians to dismiss the evidence of contamination. Therefore, it is important to address the false and misleading claims made by the TGA.
Because of the number of claims that must be addressed, this post is too long for email. Please click through to read on Substack.
INTRODUCTION
In a media release titled, ‘Addressing misinformation about excessive DNA in the mRNA vaccines’ and published 18 October, the TGA states,
“The Therapeutic Goods Administration (TGA) is aware of misinformation in recent media and online reports that claim the COVID-19 mRNA vaccines are contaminated with excessive levels of DNA. This is not the case.”
“These reports are based on studies conducted by a small number of laboratories that have attempted to investigate the amount of DNA in COVID-19 vaccines.
“While the TGA welcomes and constantly reviews the latest scientific evidence about the safety of vaccines and other biotechnology products, these recent studies fail to apply the required scientific rigour expected in pharmaceutical testing. As such, the results are not robust or reliable, and are creating confusion and concern regarding the safety of vaccines.”
These are serious allegations apparently intended to discredit scientists and reporters. Following is a comprehensive response methodically addressing the many false and misleading claims made by TGA in its statement.
In the spirit of full transparency and clearing up any remaining confusion, the comments are open to anyone who wishes to ask a question or make a comment.
Throughout the below I reference a Science Summary and Science Summary Supplement (referred to as the Supplement from here on). These documents are addendums to a letter sent to the Prime Minister on 25 September 2024 by federal MP Russell Broadbent calling for the immediate suspension of the Covid mod-RNA vaccines until the synthetic DNA contamination and its associated risks have been properly investigated. The Supplement was issued to specifically respond to the TGA’s statement dated 18 October 2024.
Broadbent’s letter and accompanying Science Summary and Supplement are co-signed by 52 internationally esteemed scientists and academics, who are concerned that,
“Excessive synthetic foreign DNA encapsulated in lipid nanoparticles can integrate into human cells, potentially leading to genomic instability, cancers, immune system disruption, and adverse hereditary effects.”
The timing of this letter coincided with the release of a report, by Dr David Speicher, finding excessive DNA contamination up to 145 times the regulatory limit in Australian Moderna and Pfizer Covid vaccines.
Responding to this information, the local government of Port Hedland voted on 11 October to join Broadbent in calling for the suspension of the vaccines and for further investigation of the contamination issue. The Port Hedland Council has now sent written warnings to all of Australia’s 537 councils, along with health practitioners in Port Hedland.
It is online reports of the Port Hedland Council motion that the TGA is now addressing with its allegations of “misinformation.”
Russell Broadbent MP’s letter dated 25 September 2024, the Science Summary and Supplement, and the Port Hedland Council Motion are all linked at the end of this post.
This response has been compiled with the assistance of Kevin McKernan (CSO and Founder, Medicinal Genomics), Dr David Speicher (Virologist, University of Guelph), Julian Gillespie (Former barrister and advisor), Dr Ah Khan Syed (Arkmedic on Substack), Dr Jessica Rose (Immunologist and Computational Biologist, FLCCC Senior Fellow), Dr Robyn Cosford (Chair of Children’s Health Defense Australia), Dr Julie Sladden (Co-Director, Australians for Science and Freedom), and Dr Deirdre Little (GP Obstetrician).
THE SCIENCE
To date, the results of independent testing of 49 vials of Pfizer and Moderna mod-RNA vaccines from Australia, Canada, the U.S., and Germany have been published in the studies listed below. All detected synthetic plasmid DNA in levels that exceed the internationally accepted level of 10 nanograms (ng) per dose.
McKernan et al. 2023.
Preprint, 12 vials, 5 batches.
2 x Pfizer bivalent, tamper sealed, in code.
2 x Moderna bivalent, tamper sealed, in code.
8 x Pfizer monovalent, tamper sealed, expired.
Speicher et al. 2023.
Preprint, 27 vials, 12 batches.
5 x Pfizer adult monovalent, tamper sealed, expired.
3 x Pfizer adult bivalent, tamper sealed, expired.
9 x Moderna child/adult monovalent, tamper sealed, expired.
4 x Moderna adult bivalent, tamper sealed, in code.
3 x Moderna child/adult bivalent, tamper sealed, expired.
3 x Moderna adult monovalent, opened, in code.
König and Kirchner 2024.
Peer-reviewed, 7 vials, 7 batches.
4 x Pfizer, expired, tamper sealed.
3 x Pfizer, in code, tamper sealed.
Speicher 2024.
Expert witness report, 3 vials, 3 batches.
1 x Moderna, child/adult monovalent, opened, expired.
2 x Pfizer, child and adult monovalent, tamper sealed, expired.
In addition, cancer genomics scientist Dr Phillip Buckhaults shared the results of his tests on the Pfizer vaccine in sworn testimony to a Senate Medical Affairs Committee, on 12 September 2023. “The Pfizer vaccine is contaminated with DNA,” he said. Watch Buckhault’s presentation here and view the slides here.
The TGA should be familiar with all of the above preprints and peer-reviewed studies, because all are discussed in Dr David Speicher’s report on Australian Covid vaccine vials, which was supplied to the TGA on 18 September 2024. The TGA has previously provided comment on the König and Kirchner paper here.
ADDRESSING FALSE AND MISLEADING CLAIMS
Following are the TGA’s claims and purported concerns, addressed one by one.
1) Claim: “All COVID-19 vaccines approved in Australia have been rigorously assessed and meet our high standards for safety, quality, and efficacy.”
Rating: False.
Why: The Pfizer product administered to the majority of Australians was never tested in a randomised controlled trial (RCT), the TGA did not review patient level data, and key tests were not required by regulators for approvals.
Pfizer’s COMIRNATY product makes up the vast majority of Covid vaccine doses given in Australia, at almost three quarters of the 72.3 million doses administered to Australians as at 10 October 2024.
However, the primary series Pfizer vaccine was never tested at scale in an RCT as many have been led to believe.
The product tested in Pfizer’s RCT, upon which the TGA’s approvals were based, was produced with ‘Process 1’, which involved high-quality In Vitro Transcription of synthetic DNA to make the spike-producing mod-RNA.
However, to scale up production for the mass rollout, Pfizer switched to a different method, called ‘Process 2’, as detailed in Pfizer’s Nonclinical Evaluation Report. The switch to Process 2 was brought to public attention in a rapid response authored by Drs Retsef Levi and Josh Guetzkow and published in the British Medical Journal (BMJ) (2023).
Process 2 involves growing DNA plasmids (sourced with assistance from Pfizer’s Gene Therapy Program) in E. coli bacteria, from which the vaccine mod-RNA is then produced (Thorn et al. 2022). The plasmids are then degraded using an enzyme called DNase and filtered out, with regulatory allowance for residual DNA of 10 ng per dose at sizes no greater than 200 base pairs (bp) remaining in the final vaccine product.
Moderna’s commercial Covid vaccine is also produced by this Process 2 method. It is unclear if Moderna’s RCT was conducted with a Process 1 or Process 2 product.
Almost all of the 43,448 participants in Pfizer’s RCT received the Process 1 vaccine, while only 252 participants received the product resulting from Process 2 - far too few to meaningfully measure safety outcomes.
Furthermore, the TGA has admitted that it “does not hold Individual Level Patient Data” in relation to provisional registration of the Pfizer vaccine, according to a response to a Freedom of Information request (FOI 2889).
This means that the TGA approved the Covid vaccines based on the manufacturers’ summaries of their own trial data. This is concerning, given allegations of fraud (summarised in this audit brief and in this book by whistleblower and biostatistician Christine Cotton) in the Pfizer Covid vaccine trial.
Carcinogenicity and genotoxicity studies were not conducted prior to approvals as stated in Pfizer’s Nonclinical Evaluation Report, and Moderna’s Public Assessment Report.
I asked the TGA for details of any testing that had been conducted looking for genomic integration events and cancers either prior to or after approval of the mod-RNA vaccines.
The TGA confirmed that, “No nonclinical studies for genotoxicity or carcinogenicity were undertaken prior to or after the TGA approval of the mRNA COVID-19 vaccines.”
This is because, “Genotoxicity and carcinogenicity studies are normally not required for the registration of vaccines according to the WHO guidelines on non-clinical evaluation of vaccines.”
2) Claim: The method of fluorometry is invalid because it overestimates DNA levels in the sample.
Rating: Misleading.
Why: Some of the labs that found excessive DNA contamination in the mod-RNA vaccines used qPCR, the TGA’s approved testing method. Several of the fluorometry tests used an enzyme called RNase A to eliminate the possibility of overestimation of DNA levels. Scientists argue that fluorometry is a more appropriate method for testing residual DNA than qPCR, which underestimates DNA levels.
The TGA states,
“Some laboratories have chosen to report DNA levels using a test called fluorometry, which is known to overestimate DNA levels in the presence of mRNA. This is because the fluorescent dye used in this test binds to both DNA - which may be present in minute amounts - and mRNA, which is the main ingredient in the COVID-19 vaccines. This leads to incorrect DNA levels being reported in these tests.”
The TGA addresses testing by “some laboratories” with fluorometry but does not mention other testing conducted with qPCR, the TGA’s preferred method for measuring residual synthetic DNA levels.
Residual synthetic DNA at levels above the regulatory 10 ng per dose limit has been found using qPCR testing by cancer genomics scientist Dr Phillip Buckhualts, genomics scientist Kevin McKernan, and virologist Dr David Speicher.
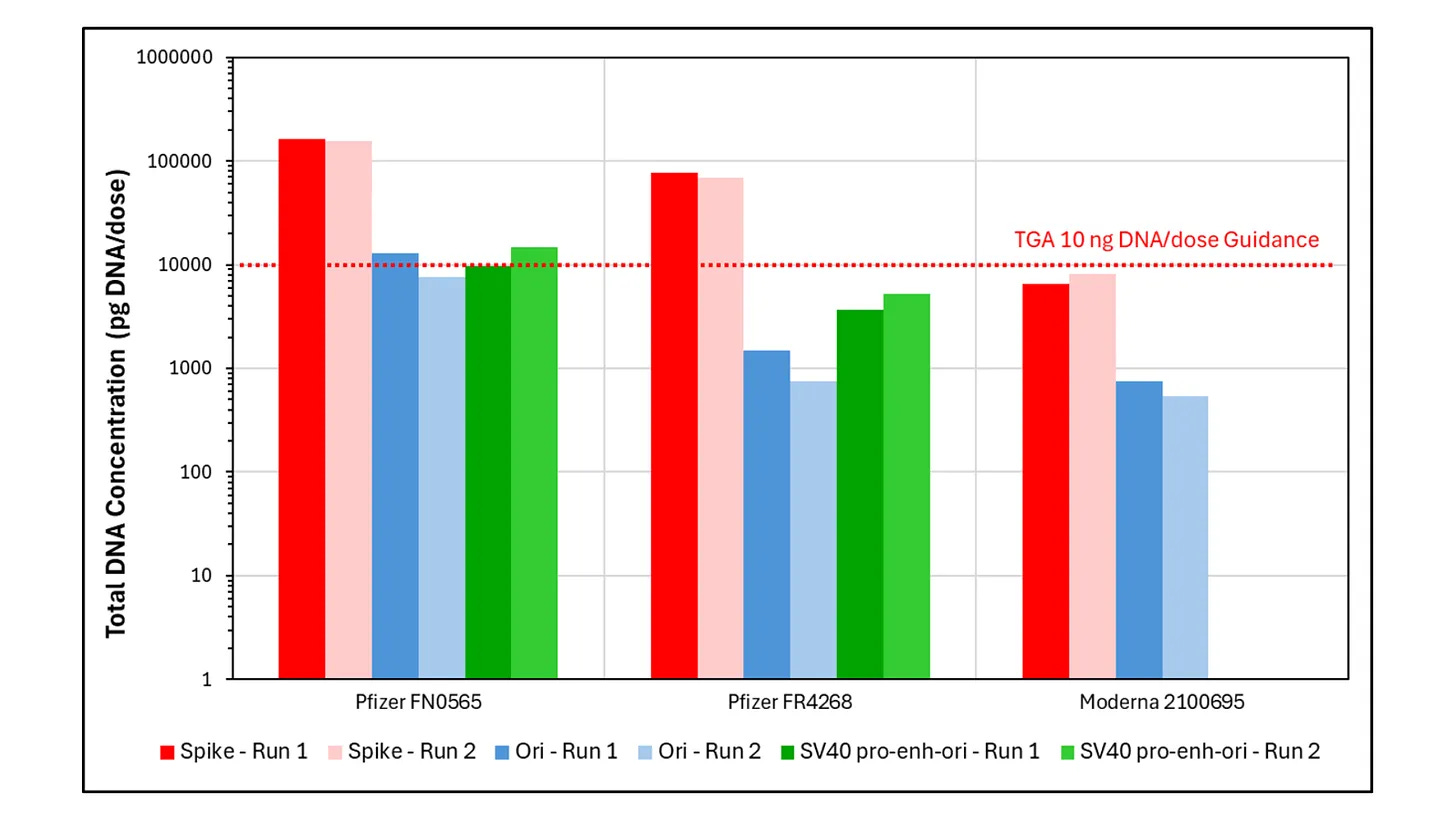
Notably, McKernan, who made the initial discovery of DNA contamination in the mRNA shots, and a gene therapy sequence called the SV40 enhancer/promoter in the Pfizer vaccine back in April 2023, found contamination at qPCR cycles much lower than what is used to detect the SARS-CoV-2 virus (McKernan et al. 2023).
The TGA states that the dye used in fluorometry can bind to the mod-RNA in the vaccine sample as well as the DNA, a problem called ‘cross-talk’, which can inflate the DNA reading. This is correct, which is why scientists have added a step using an enzyme called RNase A to degrade the RNA, thereby eliminating cross-talk.
The TGA is also correct that two studies have used fluorometry without RNase A (Speicher et al. 2023 and König and Kirchner 2024), which would result in an overestimation of residual DNA levels.
However, the TGA did not acknowledge the use of RNase A eliminating the problem of cross-talk in Speicher’s fluorometry analysis of three Australian vials and in McKernan’s fluorometry analysis of four vials (two Pfizer, two Moderna) (Speicher 2024)(McKernan et al. 2023).
Solicitor Katie Ashby-Koppens, whose law firm PJ O'Brien & Associates commissioned Speicher’s report as evidence for a lawsuit over the regulatory status of the mod-RNA vaccines, sent a copy of the report to the TGA on 18 September 2024.
Unless the TGA did not read the report, the TGA is aware of the use of RNase A to eliminate cross-talk in fluorometry tests measuring residual DNA levels in Australian vials of the mod-RNA vaccines.
It is therefore conspicuous that the TGA did not make mention of this important methodological step which directly addresses its purported concern.
As it stands, Pfizer uses fluorometry to measure mod-RNA levels and qPCR to measure DNA levels (per FOI 3390, Document 11, and FOI 3471, Document 48).
But this inconsistent cherry-picking of quantitation methods will result in over-measuring the mod-RNA, and under-measuring synthetic DNA.
McKernan says it is important to note that the dye used to measure RNA with fluorometry (RiboGreen®) has more cross-talk with DNA than the dyes used to measure DNA (such as PicoGreen® and AccurGreen®). In fact, RiboGreen® can bind to DNA with 200% more signal than RNA, meaning it will be twice as likely to detect DNA than RNA (Jones et al. 1998).
This means that when manufacturers measure RNA using the regulator-approved method of fluorometry, their RNA levels (which need to be high) will be inflated due to cross-talk with DNA. If the TGA was concerned with cross-talk, it would not allow this.
In contrast, cross-talk in fluorometry testing for DNA tends to pick up RNA signals only measured at 1-5% (Singer et al 1997). This does not mean that cross-talk should not be addressed, but rather highlights the double standard in allowing manufacturers to use methods that inflate readings of RNA (good for the manufacturer) and underestimate readings of DNA (also good for the manufacturer.)
Additionally, the dye used in qPCR testing of residual DNA will underquantify the DNA by 70% if it is fragmented with DNase 1, which is the enzyme that is used by Pfizer in its residual DNA testing (Georgiou et al. 2009).
The Supplement notes that, “The same qPCR primers [used to measure DNA levels] can measure for the modRNA, but Pfizer is choosing not to do this,” begging the question, “Why is Pfizer allowed these two different test methods?”
German scientists König and Kirchner have further criticised these “methodologically inadequate” standards because the qPCR method checks less than 1% of the plasmid DNA template, with the other 99% being extrapolated mathematically, resulting in a “massive under-detection of DNA impurities.” (2024)
This criticism was confirmed recently when the TGA released Moderna’s protocol for measuring residual DNA levels in response to my FOI request (FOI 5286). Despite most of the document being redacted, McKernan said that an unredacted detail from the protocol proves König and Kirchner correct.
“The Moderna protocol shows that they are only targeting one loci in the plasmid in the Kan gene,” says McKernan, referring to the Kanamycin resistance gene.
“We have shown that this region in some Moderna lots is 100 times lower in DNA concentration than the [synthetic plasmid] spike DNA. This variance is compounded by the inability to detect small fragments of the plasmid.”(Georgiou et al. 2009)
In other words, the TGA-approved methods may be failing to detect much of the residual DNA, especially the smaller fragments, thereby underestimating the DNA levels by up to 100-fold.
“This is why the fluorometry data, which measures all DNA, is the more comprehensive way to measure this,” says McKernan.
A final point on this issue. A patent filed by Moderna in 2014 relating to removal of DNA fragments in the mod-RNA production process specifically warns about the inadequacy of using qPCR for detecting residual DNA.
“Quantitative PCR is often applied to measure the residual DNA but it only detects the DNA molecules that contain both qPCR primers thus does not measure all other smaller DNA molecules that are partially digested,” the patent states.
This has led to the conclusion that, “the TGA appears to have been using testing methodologies… that only target a small segment of the plasmid DNA, failing to detect the bulk of the contamination, particularly those fragments under 200 base pairs where the highest risk lies,” as stated in the Supplement.
3) Claim: The tests performed do not adhere to the TGA-approved guidelines.
Rating: Misleading.
Why: There is no compendial standard for measuring DNA in mod-RNA vaccines after it has been packaged in lipid nanoparticles (LNPs). The guidelines cited by the TGA do not account for the technological development of LNPs. Further, it states that alternative methods can be acceptable if there is a scientific justification.
The TGA states,
“The guideline used by the TGA and other regulators to assess the performance of test methods is ICH Q2(R2) Validation of Analytical Procedures- external site, developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). This provides performance criteria that a test method must meet to demonstrate that its results are reliable and accurate. Using these criteria, the fluorometry method used in the quoted tests to measure residual DNA does not meet the requirement for specificity.”
As discussed in Section 2, the TGA misleadingly addresses only fluorometry testing, omitting mention of both the step taken to ensure ‘specificity’ with fluorometry (i.e.: the use of RNase A), and the qPCR testing which has also detected excessive levels of contaminant DNA.
Currently, residual DNA levels in the mod-RNA vaccines are tested by manufacturers when the vaccine is still in its soupy form, before being packaged in LNPs then decanted into vials. Regulators may then perform additional spot-testing of the final product.
There is no compendial standard for measuring DNA levels in the final vaccine product, as highlighted by Speicher in a recent interview.
The Supplement notes that, “The TGA’s reliance on outdated guidelines such as ICH Q2(R2), which do not account for the unique nature of modRNA platforms using Lipid Nanoparticles, or LNPs, has resulted in significant shortcomings.”
The aforementioned scientists who have undertaken independent analysis of residual DNA levels in the mod-RNA vaccines have discussed existing protocols, determined them to be scientifically unsuitable, and put forward reasons as to why they believe their chosen methods are better suited.
The ICH guideline cited by the TGA states that this is acceptable practice:
“Approaches other than those set forth in this guideline may be applicable and acceptable with appropriate science-based justification. The applicant is responsible for designing the validation studies and protocol most suitable for their product.”
Read a lay summary of Speicher’s methods for measuring residual DNA in the mod-RNA vaccines here.
4) Claim: “The physical reference materials were not adequately defined.”
Rating: Misleading.
Why: Key information is documented in the studies, such as tamper seal status, shelf life, and chain of custody.
After I requested clarification on this point, the TGA responded,
“A reference material has a number of purposes, including ensuring the test is working as designed, works consistently over time, and for assigning a quantitative value to the substance under test. Characterization of a physical reference material in regulatory testing involves determining and documenting the specific properties of the material to ensure it meets the required standards. This can include concentration, purity, and the shelf life.”
The reference materials in the TGA’s fully redacted FOI release of batch testing results (FOI 4558) are unidentifiable - a double standard is being applied here.
Given the TGA’s apparent ignorance of key details documented in the independent studies referenced in this article, such as use of qPCR testing, tamper seal and expiry status of samples, cold chain storage, and chain of custody, it is unclear whether the TGA has thoroughly reviewed the relevant documentation or not.
The studies to date are not written in a consistent format, as is to be expected given the different authorships, and this may have presented a challenge to the TGA in locating the information it was looking for.
Preprints that are currently being advanced through the peer-review process are likely to be refined in their presentation of materials, methods, and results.
None of these are reasons to discount the findings documented in the referenced studies.
5) Claim: Some studies only tested a small number of vials.
Rating: Misleading.
Why: Collectively, scientists have independently tested more batches than the TGA.
The TGA states that it has independently tested 27 batches of Covid mod-RNA vaccines by qPCR. Separately, by email, the TGA advised me that this testing was carried out by the TGA Laboratories Branch. It is unclear how many vials were tested across these batches.
The independent studies referenced in this article have altogether tested 49 vials across 27 batches. Additionally, Buckhaults has tested multiple batches.
If these are too few batches tested for the TGA to consider reliable, then this logic invalidates the TGA’s own independent testing, which is clearly not expansive enough for the TGA to consider reliable.
Perhaps the TGA’s concern is not with the total number of batches tested, but rather with the number of vials being tested at a time.
However, it is probable that, like the independent studies referenced in this article, the TGA’s 27 batches were not all tested at the same time either, as batches have been rolled out periodically over the past three years. Once again, the TGA’s line of argument appears to invalidate its own testing results.
Even if only a small number of vaccine vials contain excessive residual DNA, one might expect regulators charged with ensuring public safety to register concern and seek to find out if any other vials may have slipped through the net.
This should especially be the case given that particular batches have been associated with higher levels of serious adverse event reports, as identified in a Danish study published in the European Journal of Clinical Investigation (Schmeling et al. 2023). This is suggestive that a few ‘dodgy’ batches can cause a disproportionate amount of harm in the vaccinated population.
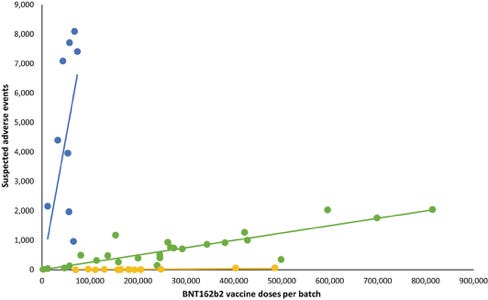
There may even be an association between highly contaminated batches and increased adverse event reporting, according to the Speicher et al. study of 27 Canadian vials.
Speicher’s co–author, immunologist and computational biologist Dr Jessica Rose, analysed reports in the Vaccine Adverse Event Reporting System (VAERS) database associated with the vaccine batches tested for residual DNA.
Rose found “preliminary evidence of a dose-response effect of residual DNA… and SAEs [serious adverse events],” which the authors said “warrant confirmation and further investigation.” (Speicher et al. 2023)
The TGA remains remarkably nonplussed.
6) Claim: “The studies also used samples that were well past their use-by date. Some samples had already been opened and used. These samples were not suitable for testing.”
Rating: False.
Why: Excessive levels of the same DNA sequences were found in vials that were both in-code and expired, tamper sealed, and opened. The use-by for administration of the products to humans is irrelevant to the ‘use-by’ of residual DNA, which remains stable at room temperature for years.
The TGA appears to be referring specifically to Speicher’s analysis of three Australian vials, all of which were expired, as all other listed studies analysed both expired and ‘in code’ vials.
Out of all the independent studies measuring residual DNA levels in the mod-RNA vaccines, most vials tested were tamper sealed, with only a handful of vials having been opened. A single vial in the Australian report had been opened.
Addressing the use-by issue first - this is somewhat of a red herring. While the mod-RNA vaccines must be kept in cold chain storage and administered to humans within a certain time frame due to the fragility of the mod-RNA and the LNPs, this is not relevant to the application of testing residual DNA levels.
In the Supplement Speicher states, “Synthetic DNA is stable at room temperature for months… DNA is so stable that you can get DNA from forensic samples (dead people buried for years) and ancient people.” (Nguyen et al. 2018)
This point was also emphasised by Buckhaults in his testimony to the South Carolina Senate.
“Moreover, there is no means by which the synthetic DNA quantities in these vials could have increased after the dry ice had evaporated. DNA does not and cannot spontaneously multiply in vaccine vials,” says the Supplement.
Though some LNPs may not be intact and, following, mod-RNA may have degraded, this would not significantly affect the DNA in the vials, says the Supplement.
The document clarifies that the only effect one could reasonably expect to find (and a minor one at that) would be that some of the DNA fragments may have broken down into smaller fragments. This would not change the overall amount of DNA in the vials and if anything will lead to an under-estimation of the DNA, not an overestimation, as shown by Georgiou et al.(2009)
Furthermore, even if DNA were to degrade (which is unlikely), the level would only go down and not up.
Therefore, by the TGA’s argument, this would suggest that Speicher’s measurements of DNA were, if anything, a lower bound of what is in the vials.
The TGA is correct that the Australian Moderna mod-RNA vaccine vial in Speicher’s analysis had been opened. A mix of used and tamper sealed vials were also tested in McKernan et al. (2023) and Speicher et al. (2023). All of König and Kirchner’s vials were tamper sealed (2024).
The used Moderna vial from the Australian analysis was a leftover with some contents remaining, retrieved from the storage facility of an Australian health practitioner, which is why it was not tamper sealed. The vial’s chain of custody is documented.
The open vial tested positive for the same DNA sequences as the tamper sealed vials, as was also the case in Speicher et al. (2023).
The TGA appears to be implying that open vials may have been tampered with in such a way as to confound results.
This would require that a person tampering with a used vial would have to interfere with the chain of custody to contaminate the vaccine with the exact same series of DNA sequences detected in the other independent studies and confirmed by regulators around the world. This is an unlikely conspiracy theory that McKernan refers to as the ‘elf on the shelf’.
7) Claim: The provenance of the samples is also not clear, so the TGA can’t know where they came from or how they have been handled.
Rating: Misleading.
Why: The TGA could easily have obtained chain of custody documentation as relates to the Australian report by contacting Speicher or replying to Ashby-Koppens’ email. Multiple analyses of the vials have documented chain of custody.
The TGA states,
“The provenance of the samples is also not clear. This means that significant information is not known about the vials used:
- where the vials were sourced
- their location, custody, or temperature before or during testing.”
As regards Speicher’s report on the Australian vials, the TGA is correct that it does not contain the chain of custody, being that it is written in the format of an expert witness report. However, PJ O’Brien & Associates advised that the chain of custody is documented in the prosecution brief pertaining to the associated lawsuit, and is available upon request.
I was able to confirm this myself when I reported on Speicher’s findings by simply sending an email to ask.
Solicitor Ashby-Koppens said that despite her notifying the Department of Health, the Minister for Health, and the TGA of Speicher’s findings more than a month ago, “To date we have never received a reply let alone acknowledgement of the letters and concerns raised therein.”
Other analyses of mod-RNA vaccines with chain of custody include: Speicher et al. 2023and König and Kirchner 2024.
As pointed out by Buckhaults in his South Carolina Senate address, perverse incentives dictate that scientists are generally unwilling to undertake politically risky research such as that discussed in this article.
By the same token, it is understandable that some health practitioners have preferred to supply vaccine vials to researchers anonymously rather than have the chain of custody trace back to their practice.
This is especially so in Australia, where the medical regulator, AHPRA, investigated and/or suspended Australian health practitioners for actions or rhetoric that could be seen to undermine the government’s Covid vaccine rollout, per its March 2021 position statement.
Regarding queries over the temperature of tested vials, the only analysis in which this has presented an issue is in Speicher’s study of three Australian vials, which were shipped on dry ice. Though the vials were cool when they arrived at his lab, the dry ice had evaporatedand no temperature was recorded at handover.
As previously discussed in Section 5, this is unlikely to have had any perceptible effect on the DNA in the vials.
Speicher said that once the vials from Australia arrived in Canada, “the shipping container was opened by me, documented with time and date, and placed in a secure fridge that can only be accessed by myself.”
8) Claim: The accreditation status of the laboratories is unknown.
Rating: Misleading.
Why: The TGA has not contacted any of the scientists to request particulars of lab status. Unknown lab status is not a reason to ignore findings with potentially grave implications for the health and safety of millions of Australians. If the TGA finds the independent testing to be unsuitable, the TGA should publish the methods and results of its independent testing in approved labs for public review.
The TGA states,
“The accreditation status of the laboratories is unknown. There is no evidence that these laboratories have Good Manufacturing Practice (GMP) certification, which is required by laboratories to perform approved testing for pharmaceutical companies. Nor do the laboratories appear to have accreditation to the international standard ISO/IEC 17025 : General requirements for the competence of testing and calibration laboratories. These types of accreditations ensure that the results they produce are robust and reliable.”
None of the scientists who have published their findings of excessive DNA contamination in the mod-RNA vaccines have been contacted by the TGA. The TGA did not respond to notification of Speicher’s report on the Australian vials.
“The work was done in a research laboratory following good laboratory practice and shows important preliminary findings on the vials that need to be confirmed by an independent lab under forensic conditions,” said Speicher of his Australian analysis, which was conducted in a certified biological level 2 laboratory attached to the University of Guelph.
McKernan is a top-tier genomics scientist who previously led an R&D team on the Human Genome Project and runs a successful medicinal genomics lab in Boston, which the TGA can learn more about here.
Buckhaults undertook his testing in his lab at the University of South Carolina. König’s work was performed at a diagnostic lab called Magdeburg Molecular Detection in Germany.
Importantly, the Supplement emphasises that the above-named scientists “have remained transparent with their work and methods at every step.”
“On the other hand, and by contrast, the TGA are entirely opaque and have been hiding their methods through redactions from the public and science community.”
This is seen in the complete redaction of FOI 4558, documenting residual DNA batch testing, and the substantive redaction of FOI 5286, documenting Pfizer and Moderna’s protocols for testing residual DNA levels in their mod-RNA Covid vaccines.

“The purpose of GMP and ISO accreditation is to ensure reproducibility and transparency,” said McKernan.
“While reproduction has occurred with transparent protocols by Speicher, McKernan, König, and Buckhaults, no such reproduction or transparency is offered by the TGA.”
Finally, from the Supplement, “As a public science agency financed by the public, it is incumbent upon the TGA to present any science they possess for being able to unequivocally refute [independent findings of excessive DNA contamination] for allaying any public fears.”
9) Claim: DNA is an approved starting material for many biotechnology products. Medicines produced by this technology have been used safely for over 40 years. Residual DNA is to be expected and is safe within the limits set by regulators.
Rating: Misleading.
Why: Therapeutics using mod-RNA packaged in LNPs are a novel development never used at scale in humans prior to the Covid vaccine rollout. Current regulations are for ‘naked’ residual DNA, not DNA carried into cells by LNPs. Residual DNA in any amount carried into cells by LNPs poses a safety risk. The Pfizer vaccine residual DNA contains a DNA sequence called the SV40 enhancer/promoter which poses a unique risk for genomic integration and cancer formation.
The TGA states that,
“DNA is an approved starting material for many biotechnology products,”
and,
“Medicines produced by biotechnology have been used by millions of patients for over 40 years. In that time, medicines containing residual DNA quantities under the required limits have presented a very low risk to human safety.”
It is true that biologics made with DNA plasmids are not new.
However, mod-RNA biologics with LNPs marketed as ‘vaccines’ for mass administration are new.
There are two key differences that the TGA has glossed over in its misleading characterisation of the novel mod-RNA vaccines, which use LNPs, as being like traditional vaccines and other biotechnology products, in which some residual ‘naked’ DNA is allowed.
First, as highlighted by König and Kirchner, DNA plasmid products typically require separation of residual DNA from proteins (2024). This is much easier to achieve than separating DNA from mod-RNA, as both are nucleic acids and therefore far more difficult to separate.
Second, while naked DNA has a half-life of 10 minutes and is quickly destroyed in the body, residual DNA in the mod-RNA Covid vaccines is not naked - it is packaged in LNPs.
According to Australia’s Gene Technology Regulator, the LNPs carrying the mod-RNA and residual DNA “transfect” the cells, meaning they “‘enter into a cell with transient expression of a protein.”
Pfizer’s biodistribution data provided to the TGA shows that the LNPs enter the blood and every major organ system, including the ovaries, testes, lymph nodes, heart, brain, liver, kidneys, and spleen.
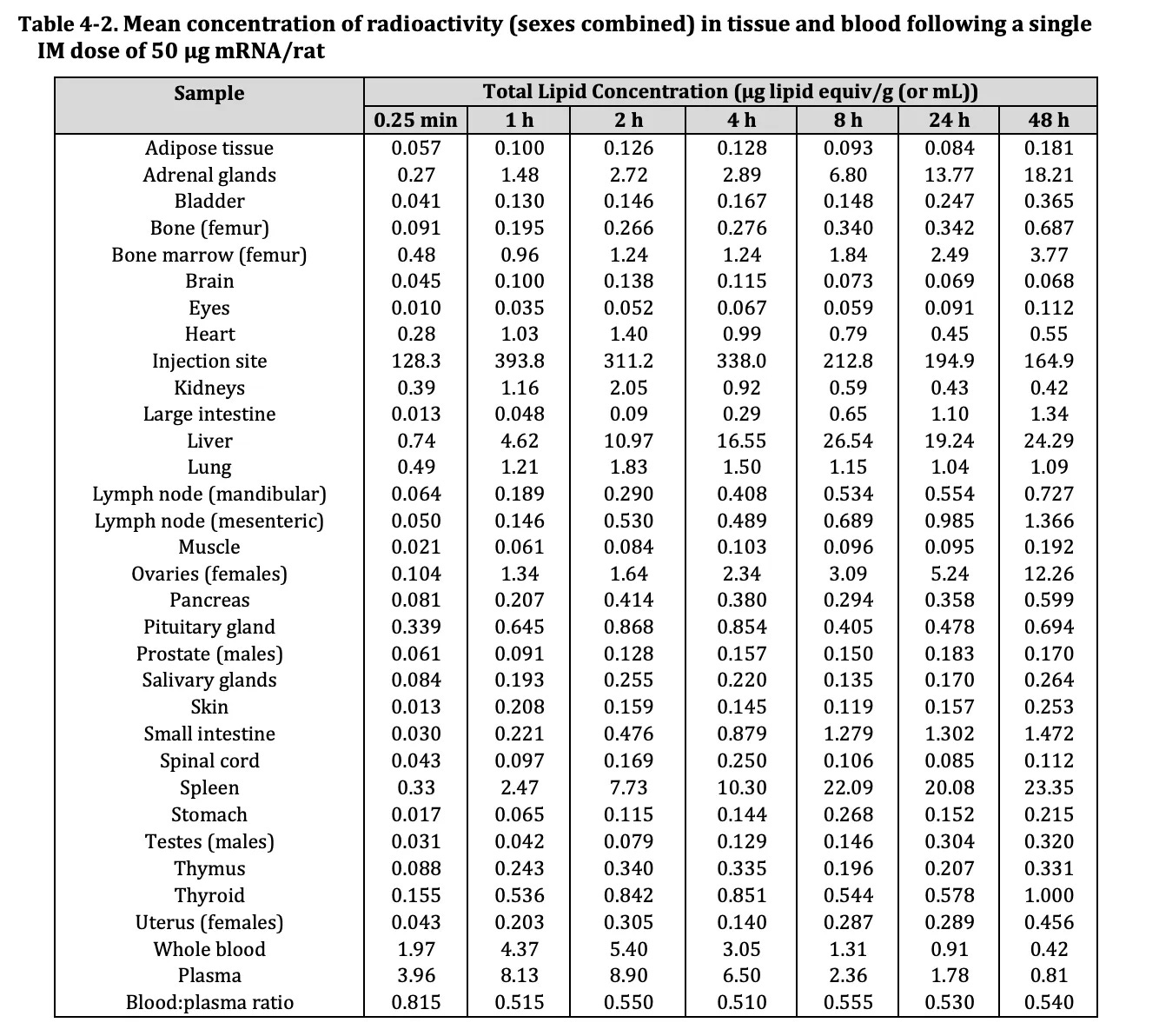
Having determined that the residual synthetic DNA in the Covid mod-RNA vaccines is being ferried into cells all over the body in its LNP packaging, let us review the risks, as outlined by a regulator and a manufacturer.
The oncogenic and genotoxic risks of residual plasmid DNA are described in industry guidance issued by the TGA’s American counterpart, the Food and Drug Administration (FDA) (emphasis mine),
“Residual DNA might be a risk to your final product because of oncogenic and/or infectivity potential. There are several potential mechanisms by which residual DNA could be oncogenic, including the integration and expression of encoded oncogenes or insertional mutagenesis following DNA integration. Residual DNA also might be capable of transmitting viral infections if retroviral proviruses, integrated copies of DNA viruses, or extrachromosomal genomes are present.”
A patent filed by Moderna in 2021 also points to the integration risk of residual DNA packaged in LNPs. It states (emphasis mine),
“There are multiple problems with prior methodologies of delivering pharmaceutical compositions in order to achieve effective protein expression for both therapeutics and bio-processing applications. For example, introduced DNA can integrate into host cell genomic DNA at some frequency, resulting in alterations and/or damage to the host cell genomic DNA.Alternatively, the heterologous deoxyribonucleic acid (DNA) introduced into a cell can be inherited by daughter cells (whether or not heterologous DNA has integrated into the chromosome) or by offspring.”
The same patent details the oncogenic risk of not adequately removing residual DNA:
“The DNA template used in the mRNA manufacturing process must be removed to ensure the efficacy of therapeutics and safety, because residual DNA in drug products may induce activation of the innate response and has the potential to be oncogenic in patient populations.”
These risks are also detailed in the aforementioned Moderna patent filed in 2014.
The above-stated risks should be unsurprising - the risk of integration of plasmid DNA integration into the natural DNA of mammalian cells was demonstrated as early as 1982 (Southern and Bern 1982).
The Science Summary states that, “The presence of foreign synthetic DNA in the cytoplasm alone [i.e.: inside the cell] induces cancer.” This claim rests on citations of peer-reviewed evidence published in the Frontiers of Immunology (He et al. 2023) and the American Association for Cancer Research journal Cancer Discovery (Kwon et al. 2020).
The TGA provides no citations for its claim that biologics made with DNA plasmids present “a very low risk to human safety.”
Further evidence for risks of cancer and genomic integration in particular will be discussed in Sections 11 and 12.
Finally in this section, the synthetic DNA in the Pfizer vaccine contains a sequence called the SV40 enhancer/promoter (two overlapping sequences, the enhancer and the promoter) which poses a unique risk for genomic integration and cancer formation.
As explained in the Science Summary, adjacent to the SV40 sequences (which are not the same as the SV40 whole virus) found in the Pfizer product is “an internal [mammalian] origin of replication that can potentially cause copies of the synthetic DNA to be made inside human cells.” (emphasis original)
This is significant because, “Any additional copies of the synthetic DNA generated would amplify the risk of genomic integration with human DNA and increase the risk of malignant tumours (cancers) associated with the SV40 virus.” (Rotondo et al. 2019)
This replication could occur in anyone who has the SV40 virus itself or the human BK or JC polyomaviruses. While SV40 infections are uncommon, BK and JC viruses are prevalent and often asymptomatic. (DeCaprio and Garcia, 2013) (Hussain, et al. 2020)
“Any additional copies of the synthetic DNA generated would amplify the risk of genomic integration with human DNA and increase the risk of malignant tumours (cancers) associated with the SV40 virus,” states the Summary. (Rotondo et al. 2019)
The Science Summary explains that in any given Pfizer vaccine, there will be billions of SV40 molecules. But, “only 3-10 copies of this synthetic DNA containing the SV40 enhancer are needed to be inserted into a single cell for the risk of insertional mutagenesis (cancers) to exist.” (Dean, et al. 1999)
This SV40 enhancer has also recently been reported to be a Somatic Hypermutation Targeting element which can induce mutations in the host cells it enters (Šenigl et al. 2024).
“Scientists have demonstrated that plasmids with SV40 mammalian origins of replication (like those found in the Pfizer vaccine) can replicate in mammalian cells in absence of SV40 Large Tumor Antigen,” said McKernen. (Madzak et al. 1989) (Giannakopoulos et al. 2009)(Piechaczek et al. 1999)
This is significant because fact-checkers sometimes cite the absence of SV40 Large Tumor Antigen to insist that the SV40 enhancer is safe.
“Replication of plasmid DNA in mammalian cells may explain why have all detected residual spike nucleic acid longer than the promised 48 hours post injection, even up to 60 days after vaccination,” said McKernan. (Castruita et al. 2023)(Krauson et al. 2023) (Röltgen et al. 2022) (Hanna et al. 2023) (Gonzalez et al. 2023)
Furthermore, the SV40 promoter sequence is known to bind to tumor suppressor protein p53 (also called the Guardian of the Genome for its important role in DNA damage response and repair), leaving insufficient p53 to protect against cancer formation (Drayman, et al. 2016).
The cancer risk of inhibiting p53 is discussed further in my articles, ‘Two new studies suggest mRNA Covid vaccines can contribute to cancer formation,’ and ‘Forced retraction of Covid vaccine cancer-risk study, scientist alleges.’
Interestingly, it appears that regulators were just as much in the dark on the presence of the SV40 enhancer/promoter in the Pfizer vaccine as the public.
In emails released under Freedom of Information (FOI) in Canada, a senior Health Canada (HC) official, Dr Dean Smith, admitted that the presence of the SV40 enhancer/promoter sequence in the Pfizer vaccine was not disclosed to regulators prior to obtaining emergency use approvals.
“Pfizer has communicated to us recently, that they apparently chose not to mention this information to EMA, FDA or HC at the time of their initial or subsequent submissions,” wrote Smith to the FDA in an email on 23 August 2023.
This is four months after McKernan first discovered the sequence in the Pfizer vaccine, and two months after McKernan presented his findings to the FDA.
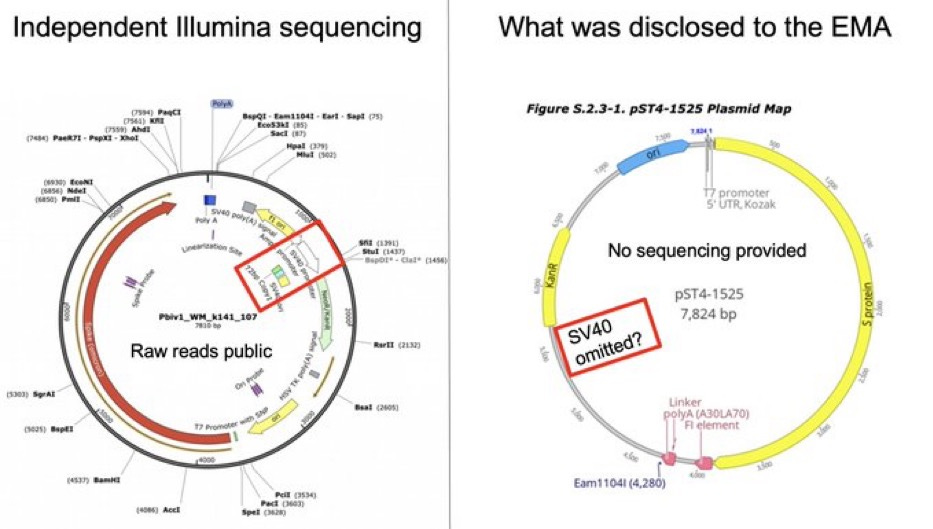
Though the TGA is not mentioned in Smith’s email, it is improbable that Pfizer disclosed the presence of the SV40 enhancer/promoter sequence to the TGA only, and not to the other mentioned international drug regulators.
The Australian Minister for Health, Mark Butler, has since stated in response to questions on notice tabled in January 2024 that,
“The TGA and the Department do not consider the presence of the SV40 promoter to be a safety concern. The SV40 promoter sequence is considered safe and is a common sequence used in biotechnology-based medicines.”
It is curious then, that HC is working to get the SV40 enhancer/promoter removed from Pfizer’s vaccine.
A draft Clarifax response emailed from Senior HC Biologist/Evaluator Michael Wall to HC Manager of Vaccine Quality Tong Wu on 29 August 2023 states, “Health Canada will continue to work with international regulator partners to achieve harmonization regarding removal of these sequence elements from the plasmid for future strain changes.”
Why would a regulator want to remove a ‘safe and common sequence’ from the Pfizer vaccine?
10) Every batch of mod-RNA Covid vaccines is compliant with the regulatory guidelines for residual DNA concentration.
Rating: Misleading.
Why: Independent testing of Australian vials found residual DNA in the mod-RNA vaccines at levels of seven to 145 times the allowable limit. Manufacturers are allowed to test their own products with methods that will underestimate the amount of DNA in the vials. The TGA has tested only a fraction of the total number of batches rolled out to Australians in the past three and a half years.
The TGA states,
“Every final batch of the mRNA COVID-19 vaccines released in Australia has met the regulatory requirements for residual DNA concentration. To date, the TGA has also independently tested 27 batches of COVID-19 mRNA vaccines by qPCR to confirm the residual DNA concentration in the final product. The vaccines met the required limits for residual DNA.”
The TGA has not sought to verify or falsify independent findings by Speicher which contradict this statement. Speicher detected synthetic plasmid DNA contamination in Australian vials of Pfizer and Moderna Covid vaccines at levels of between seven to 145 times the allowable limit (including lots for children) as detailed in his report, which was provided to the TGA on 18 September 2024.
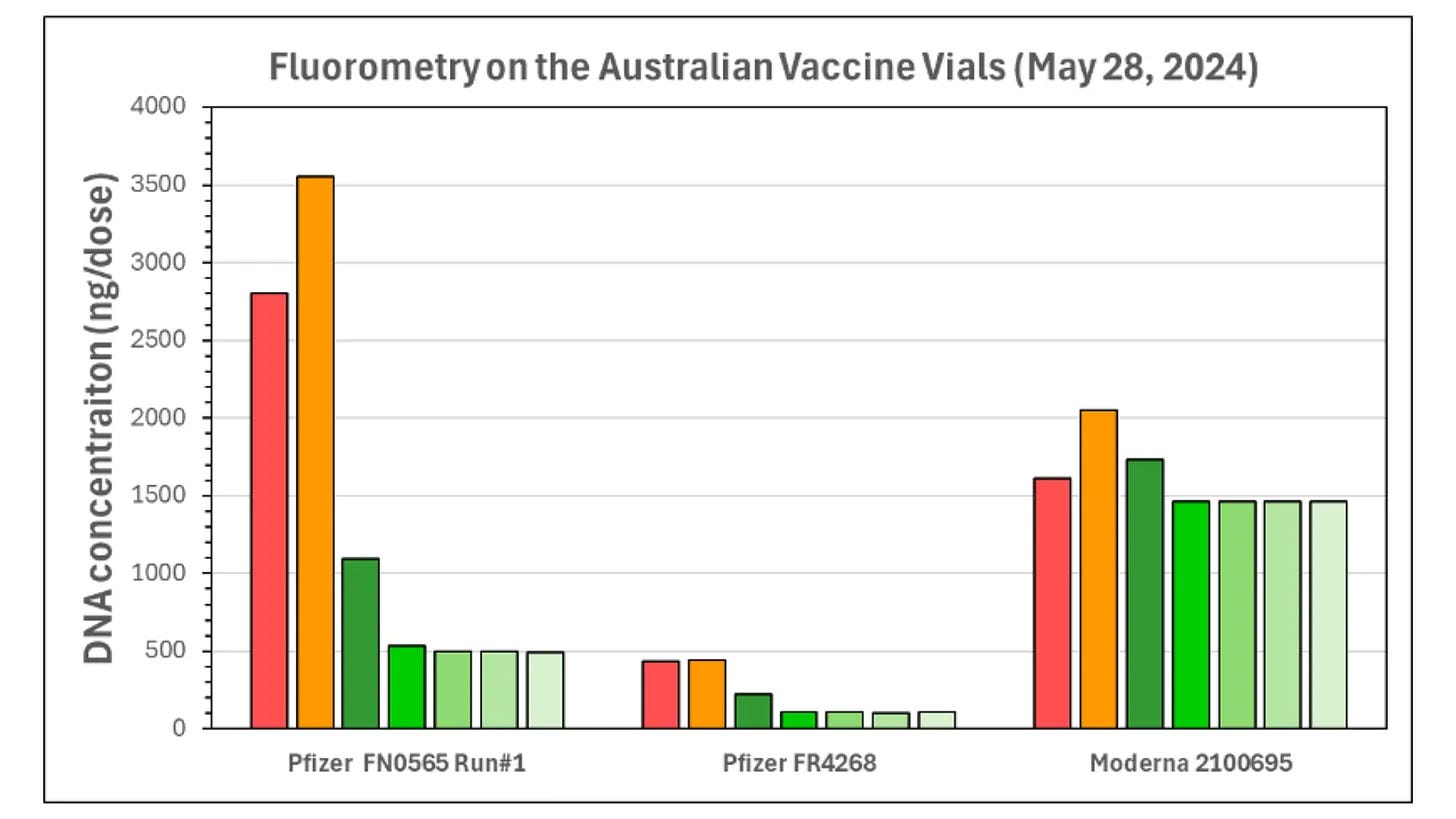
As discussed in Section 2, the TGA allows manufacturers to test DNA levels with methods that will underestimate the amount of DNA in the products.
Out of 72 million doses administered to Australians across 429 batches, only 27 batches have been tested by the TGA. This represents a small fraction (6%) of the lots rolled out to Australians.
That said, as discussed in Section 9, the regulatory limits are inappropriate, as they do not account for LNPs, and the presence of the gene therapy SV40 enchancer/promoter sequence in the Pfizer vaccine.
11) Claim: “To date, neither the TGA nor any international regulator has established a causal link between Covid vaccines and any type of cancer.”
Rating: Misleading.
Why: Neither the TGA nor any international regulator has looked for a causal link between Covid vaccines and any type of cancer.
The TGA says that it has not established a causal link between Covid vaccines and any type of cancer. However, it has never provided any evidence that it looked for such a link and found a negative result.
The TGA did not require carcinogenicity testingprior to approving the mod-RNA Covid vaccines. As discussed in Section 1, the TGA confirmed to me via email that it has not conducted any testing for carcinogenic effects after the approval process, either.
Moreover, it appears that the TGA has not been monitoring cancer incidence in the format stipulated in the provisional approval conditions for the Covid mod-RNA vaccines.
An FOI request (FOI 5275) seeking monthly cancer incidence surveillance data relating to the Pfizer vaccine rollout was denied by the TGA because the documents requested “do not exist.”
These documents were requested because, as stated in the Pfizer AusPAR, “the sponsor is required to submit monthly safety summary reports for the first 6 months of registration, and thereafter at intervals specified by the TGA.”
However, “The reason the documents do not exist is because the TGA does not hold ‘incidence data’ related to cancer,” stated the TGA response.
There is evidence suggesting a causal link between Covid vaccines and cancer, although this is outside of the scope of the issue at hand, which is the relationship between synthetic DNA contamination in the mod-RNA shots, and cancer.
The scientific evidence discussed in Section 9, especially about the role of the SV40 enhancer/promoter in cancer formation, is enough to suggest that regulators ought to investigate the issue.
The necessity for investigating the cancer link was highlighted by internationally lauded Professor of Oncology Angus Dalgleish at the Port Hedland Council meeting on 11 October 2024 that triggered the media cycle precipitating the TGA’s statement.
In a video recording for the Port Hedland Councillors (which can now be viewed here), Dalgleish said that he had seen an upsurge in aggressive, fast-progressing cancers in his oncology practice, particularly colorectal and blood cancers, amongst patients who had received Covid vaccine boosters.
Having previously sat on the scientific board of mRNA technology company CureVac, Professor Dalgleish, who is the lead co-signatory on Broadbent’s letter dated 25 September to the Prime Minister, emphasised that he is well-placed to understand and speak to the risks and benefits of the Covid mod-RNA vaccines, which he said are more properly called a gene therapy.
“We need our health authorities to begin monitoring these trends, develop testing protocols for those exposed to synthetic DNA contamination, and prepare treatment pathways for the inevitable rise in vaccine-induced conditions,” he said.
Research scientist Dr David Wiseman presented statistical evidence corroborating Dalgleish’s anecdotal experience to the FDA in June of this year, concluding that “modRNA vaccines cannot be ruled out” as a causal factor in Covid era cancers (Wiseman et al. 2024).
It is too early to identify the prevalence of cancer diagnoses in Australia because the Australian Institute of Health and Welfare(AIHW) has not published the actual figures since 2020.
This is because the AIHW has to wait for all the states and territories to provide the data, to produce the national figure, a media spokesperson advised fellow Substacker Alison Bevege.
However, the lack of data from the AIHW need not stop the TGA from investigating those who have already reported adverse events following immunisation (AEFIs), especially relating to cancer, to its safety surveillance database.
As at 4 October 2024, the TGA had received almost 300 reports of cancer-related AEFIs associated with the Pfizer and Moderna Covid vaccines, according to data aggregator site OpenDAEN.
While these reports likely represent a fraction of the cancers experienced after vaccination in Australia due to the known under-reporting factor and lack of awareness among health practitioners of cancer as a potential AEFI, these several hundred cases provide opportunity for investigation.
Dr Rado Faletič, a Pfizer Covid vaccine-injured scientist and Founder/Co-Director of Australia’s peak body for the Covid vaccine-injured, COVERSE, has repeatedly criticised the TGA for failing to follow up on AEFI reports, which present an opportunity for commissioning tissue biopsies and other tests to look for causal mechanisms.
“The TGA are practising no science (zero follow-up of AEFI reports), old science (outdated quality assurance techniques), & secret science (fully redacted disclosures). This is incredibly disrespectful towards those of us whose lives have been destroyed by the Covid vaccines,” Faletič posted recently on social media.

The TGA confirmed to me via email last year that it does not attempt to confirm causality for the majority of AEFIs reported to its safety surveillance database.
Anecdotally, I have never met a Covid vaccine-injured Australian who has been contacted by the TGA to initiate investigation after reporting an AEFI - and I have conducted scores of interviews with Covid vaccine-injured Australians while volunteering for Jab Injuries Global, a selection of which are documented here.
Ultimately, regulators’ claims to have not established a link between Covid vaccines and cancer is no kind of assurance at all when viewed in historical perspective, notes Dr Robyn Cosford, a Professor of Nutritional and Environmental Medicine.
“It took regulatory authorities some 40 years to recognise a link between tobacco and lung cancer,” said Dr Cosford.
This was despite evidence from animal studies and statistical analyses suggesting a causal link long before it was officially acknowledged by the US Surgeon General, in 1964 (Lickint et al. 1955)(Müller et al. 1940)(Doll et al. 1950)(Croninger et al.1958)(US Department of Health, 1964).
“It is interesting to note the financial incentives for this causation to not be acknowledged readily: at the time, cigarette smoking was a significant part of the American culture, and the tobacco industry was a significant component of the US economy,” said Dr Cosford (Stull 2009)(Brawley et al. 2013).
Food for thought, given Australian State and Federal Government investments into mod-RNA vaccine research and manufacturing in Victoria with Moderna, and in Queensland with Sanofi, and the fact that the TGA receives 96% of its drug regulation funding from industry (Demasi, 2022).
12) Claim: There is no evidence of mRNA vaccines or biological medicines used in Australia resulting in integration of residual DNA into human DNA genome.
Rating: Misleading.
Why: The TGA has not looked for genomic integration. There is putative evidence of genomic integration of residual DNA in vaccines outside of Australia. There is also evidence pointing to the strong possibility of genomic integration, which should spur the TGA to look for it.
The TGA states,
“There has been no evidence of mRNA vaccines or biological medicines used in Australia resulting in integration of residual DNA into the human DNA genome. This includes products such as insulin, which are injected multiple times a day for life-long treatments.”
There are several sleights of hand at work here.
First, there is no evidence that the TGA or any other regulator has looked for genomic integration events associated with insulin products.
Second, although LNPs are a new innovation in insulin products, neither LNPs nor mod-RNA are not known to be routinely used in standard injectable products (Muntoni et al. 2019)(Liu et al. 2023).
As mentioned previously, separating DNA from proteins is easier due to the substantial differences in these molecules. Separating DNA from mod-RNA, which is complementary and bound to the DNA, is very challenging.
Third, there is no evidence of mod-RNA vaccines in Australia resulting in integration of residual DNA into the human DNA genome because no one has looked for it.
As discussed in Section 1, the TGA did not require genotoxicity testing prior to approving the mod-RNA Covid vaccines, nor has the TGA conducted any genotoxicity testing since.
When I previously asked the TGA for the evidence on which it based its claim that mod-RNA Covid vaccines do not enter the nucleus or integrate with human DNA, the TGA sent me a Mayo Clinic fact page with no links to studies or scientific evidence of any sort.
The TGA also pointed me to an RMIT ABC Fact Check post from 2022 purporting to ‘debunk’claims that mRNA jabs are genotoxic. This is the same site that ‘debunked’ claims that Covid vaccines can cause menstrual disruption, before peer-reviewed scientific studies proved that they can and do (the site has still not been corrected) (Edelman et al. 2022)(Nazir et al. 2022).
In 2023, RMIT ABC Fact Check was suspended by Facebook after allegations of bias in its coverage of the Voice debate, and the fact-checker’s failure to maintain its registration with the International Fact-Checking Network (IFCN).
The TGA did provide one commentary article published in a science journal (Merchant, 2022) which discussed a well-known study I had previously sent to the TGA showing an in vitro (in a dish) finding that Pfizer mod-RNA had reverse transcribed into the DNA of a liver cell line (Aldén et al. 2022).
Merchant worried that Aldén’s finding “may be detrimental to public confidence in mRNA therapeutics in general if not proven in vivo” (in humans) and emphasised that the in vitro finding could not be extrapolated to generalise about what might be happening in humans.
While this point has prompted independent scientists to undertake in vivo testing in an effort to replicate or disprove in vitro findings, the TGA appears to have taken this opinion article as confirmation that no further testing is required.
A fact page, a discredited fact-checker, and an opinion article: That is the entirety of the evidence that the TGA has offered to support its position that vaccine contents don’t enter the nucleus or genomically integrate.
Fourth, a key assumption underlying claims that synthetic plasmid DNA cannot enter the cell nucleus, and cannot genomically integrate with host DNA, is that the contaminating DNA will never make it into dividing cells, which would be required for integration to occur.
This in turn is based two false assumptions:
That the vaccine contents are broken down quickly by the body anyway, so will never make it into dividing cells. The Australian Health Department still states that “the mRNA is broken down quickly by the body,” on its Covid vaccine fact page. This is despite scientific evidence showing persistence of vaccine nucleic acids (which could be mod-RNA or DNA) in vaccinated people for up to 28 days in plasma, 30 days in heart tissue, and 60 days in lymph nodes (Castruita et al. 2023)(Krauson et al. 2023) (Röltgen et al. 2022).
That the LNPs containing both mod-RNA and contaminant DNA mostly stay in the muscle at the injection site, a myth that was spread by regulators and health officials despite Pfizer’s biodistribution data showing that this was not the case. As muscle cells do not divide, there’s no problem, the logic goes. But, while it is true that muscle cells don’t divide, LNPs distributed around the body can transfect any number of dividing cells in various organ systems.
Once inside a dividing cell, it’s only a matter of time before the LNP contents get into the cell nucleus, McKernan said earlier this year.
“In any dividing cell, the nucleus dissolves. So, when people say the DNA can get into the cytoplasm [inside the cell membrane] but won't get into the nucleus, well, in any dividing cell, it will end up getting into the nucleus.”
Now recall the Office of the Gene Technology Regulator’s advice that the mod-RNA vaccine contents do indeed transfect (enter) cells, and that statement takes on a weight of new meaning.
Independent of cell division, the SV40 enhancer/promoter is a known nuclear targeting sequence used for gene therapy, as discussed in Section 9. SV40 enhancer/promoter plasmids were developed specifically to transfect non-dividing muscle cells, as they are very effective nuclear targeting sequences (Vacik et al. 1999).
It is significant that the TGA denied the existence of evidence that mod-RNA vaccines in Australia are resulting in integration of residual DNA into the human DNA genome because there is early evidence of integration in tests outside of Australia.
As I reported in March of this year, McKernan has detected vaccine contaminant DNA in ovarian cancer cell lines treated with Pfizer’s Covid vaccine in an experiment conducted together with German molecular biologist Dr Ulrike Kämmerer.
The scientists found a chimeric combination of human ovarian cell line DNA and spike sequence DNA derived from the contaminating plasmid at at least one, and possibly two sites, which is suggestive of DNA integration.
“If anything, this work has put to bed the question regarding if this contaminant DNA gets into the cell, and the chimeric human and contaminant spike DNA sequences imply it has entered the nucleus,” McKernan said at the time.
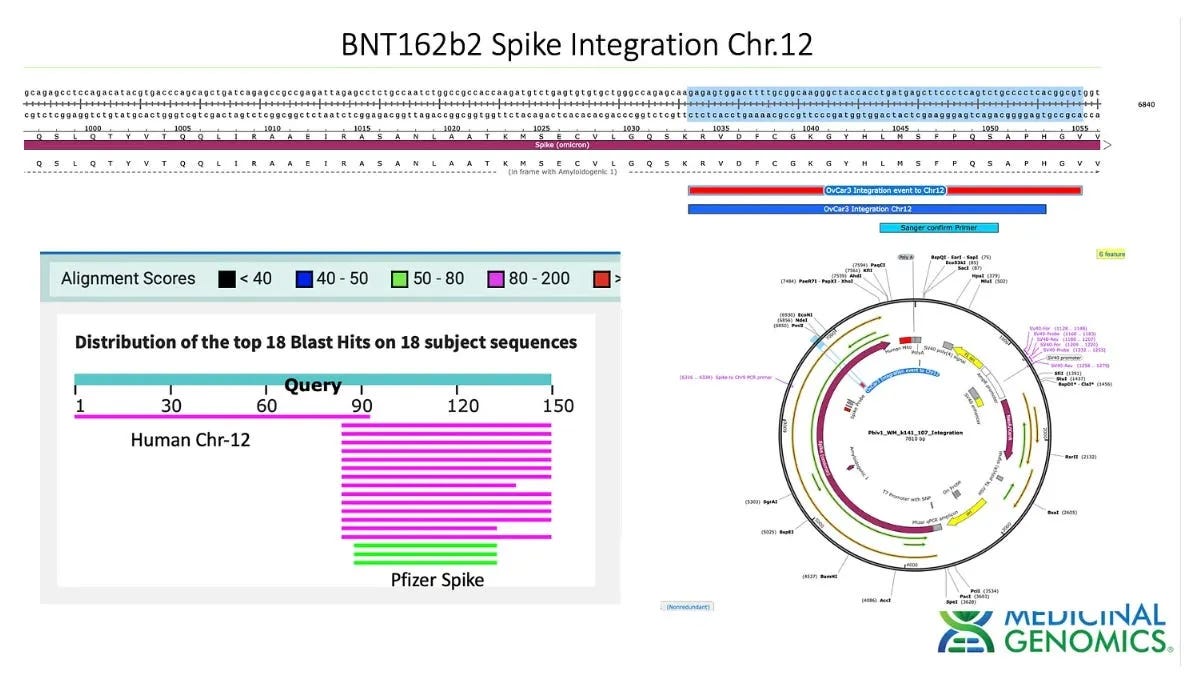
Just this month, McKernan posted early results of further testing, which appear to similarly show integration events; however, this work is not yet written up in full, so it will not be discussed further here.
Relatedly, several young women who suffered adverse effects after Gardasil vaccination (including one death) tested positive for HPV DNA that was not the result of HPV infection, leading to speculation that residual DNA in the vaccine integrated with the DNA of these victims, as reported by science journalist Maryanne Demasi.
In a bombshell follow-up, pseudonymous Substacker Arkmedic went one further, making the case that residual DNA can pose an integration risk in any vaccine which contains transfection agents such as LNPs (as already discussed), aluminium adjuvants, polysorbate, and others.
13) Claim: There were no adverse effects detected on male or female fertility, fetal deaths, birth defects, or developmental delays in the combined reproductive and development animal studies.
Rating: Misleading.
Why: These studies are not publicly accessible and so cannot be scrutinised. Documented reference to fetal harms was omitted from Pfizer’s Public Assessment Report without explanation, raising the question of whether this was an isolated incident, or not.
The TGA says,
“Furthermore, in the combined reproductive and development animal studies using 200-times the clinical dose of mRNA vaccines, there were no adverse effects on male or female fertility, fetal deaths, birth defects, or developmental delays.”
As this statement was accompanied by no citations, I emailed the TGA to request links to the relevant studies. The TGA responded with links to:
The Australian Public Assessment Report (AusPAR) for COMIRNATY™ (January 2021).
The AusPAR for SPIKEVAX™ (August 2021).
‘Nonclinical Evaluation Report, BNT162b [mRNA] COVID-19 vaccine (COMIRNATY™) (January 2021), released under FOI.
None of these documents contain animal studies. Rather, they contain summaries of animal studies.
This is problematic because without the ability to scrutinise the studies, no one can verify that the TGA’s claim is accurate. There are reasons to be skeptical.
The Moderna AusPAR states that “the effect on male fertility has not been determined.” Given that this is the only document provided by the TGA to support its claim as far as the Moderna product is concerned, it is unclear how the TGA determined this product has no adverse effects on male fertility.
The Pfizer Nonclinical Evaluation Report states that raw data from an LNP distribution study on Wister rats (Study 185350) were not provided to the TGA, indicating that, as with the human trial data, the TGA accepted the manufacturer’s summary of the study rather than reviewing the data itself. The report states that a histology picture of a rat that was “humanely killed” during the study was not provided to the TGA either.
An overview of the Pfizer vaccine released under FOI request (FOI 2389, Document 1) shows that fetal defects initially identified by the regulator were inexplicably left out of the published Pfizer AusPAR, and the safety category for the product was upgraded.
The consultation document states (emphasis mine),
“A combined fertility and developmental toxicity study in rats showed increased occurrence of supernumerary lumbar ribs in fetuses from COMIRNATY-treated female rates.”
The vaccine was issued Pregnancy Category B2, which means that, “Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage.”

In the published version of the Pfizer AusPAR report, however, the above reference to fetal harm was omitted and the Pregnancy Category was upgraded to B1, which means, “Studies in animals have not shown evidence of an increased occurrence of fetal damage.”
There is no indication given in the discussion section of the document as to why the TGA upgraded the pregnancy safety category and removed mention of fetal abnormalities.
The Pfizer and Moderna AusPARs note that despite “missing information” on use in pregnancy and while breastfeeding, this lack of safety data is considered “acceptable” by the TGA.
Furthermore, the TGA has gone to great lengths to block attempts to obtain the animal safety studies.
Tony Nikolic, Director of Sydney law firm Ashley, Francina, Leonard & Associates, subpoenaed the animal safety studies held by the TGA in 2021 for a lawsuit challenging the New South Wales Government’s Covid vaccine mandates (Kassam v Hazzard). However, the TGA refused to provide the studies.
"They spend more taxpayer funds blocking than complying,” Nikolic said of the TGA’s refusal.
FOI requests have also been blocked. After extensive wrangling with the TGA, a group called Doctors for COVID Ethics was able to obtain part of a Pfizer animal toxicity study report, but the TGA refused to release it in full for the reason that it held “commercially valuable information.”
After the request was lodged, in February 2021, the TGA initially agreed to release only 16 pages out of 1,145 total pages. Release of these 16 pages was delayed and the matter taken to the Office of the Australian Information Commissioner (OAIC). The TGA requested an extension of 180 days longer than the 30-day deadline for processing FOI requests - the OAIC granted the TGA a 60-day extension.
After a scientific article containing part of the Pfizer animal toxicity study data was published in a journal, putting the information into the public domain, the TGA eventually compromised, publishing 928 pages of the 1,145-page document to the public log, with substantive redactions (FOI 2289, Document 1).
GP Obstetrician Dr Deirdre Little requested (FOI 2565) from the TGA, in July 2021,
“All Developmental and Reproductive Toxicity Studies available to the TGA regarding the Pfizer and AstraZeneca COVID-19 vaccines including histology reports of gonads of vaccinated animals”.
Little was required by the TGA to reduce the scope of her request in August 2021. She complied, reducing it to,
“Histopathology/microscopic evaluation of gonads (ovaries/testes) of vaccinated animals in relation to Pfizer and AstraZeneca COVID-19 vaccines”.
Later that month the TGA rejected Little’s FOI, claiming it was “too voluminous to process,” despite her request having been revised down from 11 to three studies.
In September, Little initiated an internal review, which was rejected on the basis that, “Pfizer and AstraZeneca provided these studies to the TGA in confidence,” and, “the documents are likely to require numerous redactions as the documents are unpublished studies that contain information that is commercially sensitive and valuable.”
Little said that she subsequently appealed to the OAIC, however she is still awaiting a decision over three years later.
All of this may be immaterial as regards the 50 million or so doses of Pfizer vaccine administered in Australia, however, because some of the Pfizer animal studies used the Process 1 product (as detailed in the Pfizer Nonclinical Evaluation Report).
The results of animal studies conducted with the Process 1 product are irrelevant to the issue of DNA contamination, which is the result of Process 2 production.
14) Claim: “Evidence from the more than 13 billion vaccine doses given worldwide shows that COVID-19 vaccines have a very good safety profile in all age groups. The benefits of the approved vaccines far outweigh the possible risks.”
Rating: False.
Why: This is one of those Big Lies that gets repeated ad nauseum in the face of all evidence to the contrary. Several points are discussed below but it is not worth wasting time, as this claim is tangential to the main issue of scientific findings of excessive DNA contamination in the mod-RNA vaccines.
It is pretty well universally accepted at this stage that the Covid vaccines have a higher risk-to-benefit ratio for healthy children and young adults. The risk profile was acknowledged by the Australian Technical Advisory Group on Immunisation (ATAGI), for example, in its booster advice issued in March 2023:
”Adolescents and younger adults have a lower age-related risk of severe COVID-19, and a comparatively higher risk of myocarditis following vaccination.”
Currently, ATAGI does not recommend Covid boosters to healthy Australians under the age of 65.
As previously reported on my Substack, the Covid vaccines are associated with an AEFI reporting rate (rate, not raw numbers) orders of magnitude above what has ever been seen before in Australia, and regulators have previously withdrawn vaccines and other medicines for far smaller safety signals.
Moreover, this statement from the TGA suggests that the regulator takes a rather Communist view of vaccine safety. I know numerous Covid vaccine-injured people who say that the benefits did not outweigh the risks for them, but the TGA would appear to consider their suffering to be acceptable collateral damage.
There is not the space to litigate the matter of general Covid vaccine safety in this post. The TGA appears to have thrown this in as a boilerplate assurance to appease the sponsors and gin up public confidence.
CONCLUSION
In conclusion, we reject categorically the TGA’s claims that independent scientific evidence of excessive residual synthetic DNA in the mod-RNA vaccine is invalid, and that reporting on this science is “misinformation.”
The TGA should commission the necessary testing to provide evidence for its claims.
The TGA should also unredact all batch testing results to date, and make all relevant studies available for public scrutiny.
Finally, readers should consider the implications of the TGA’s use of the term “misinformation” in light of the Labor Government’s proposed legislation to combat online misinformation. A final version of the Combatting Misinformation and Disinformation Bill 2024 is due to be tabled in parliament later this year.
If passed, the legislation will require social media platforms to censor content that has been identified by authorities and accredited fact checkers to be ‘misinformation.’ This means that scientists will be unable to share their dissenting work and opinions online, and independent reporters will be unable to share their reporting on it.
The Aligned Council of Australia is running a campaign in opposition to the bill: find out more here.
LINKS
Port Hedland Motion website: https://porthedlandmotion.info/
Port Hedland Council Special Meeting (11 October 2024) Agenda (including details of the motion): https://hed.la/9jg
Port Hedland Council Special Meeting (11 October 2024) video: https://hed.la/nw6
Russell Broadbent MP’s letters to the Prime Minister dated 20 and 25 September 2024, with Science Summary and Supplement: https://russellbroadbent.com.au/australiansdemandanswers/
To support my work, share, subscribe, and/or make a one-off contribution to my Kofi account. Thanks!
The precise number of Pfizer doses administered has been requested from the Department of Health. This article will be updated when a response is received.
An additional comment from McKernan, “Speicher et al. also noted a 100-fold more spike DNA than vector DNA when using 2 different assays of the same amplicon length (2023). DNase I is known to struggle with digestion of DNA that is hybridized to RNA (RNA:DNA hybrids) as exists in the spike region of the vaccines (Sutton et al. 1997). The single Kan amplicon qPCR described by the TGA cannot possibly monitor the full complexity of the residual DNA after such a manufacturing process.”
The under-reporting factor (URF) for predominantly passive surveillance systems such as the DAEN (and affiliated state and territory systems) is estimated in the academic literature to be between 10-100 fold (Hazell and Shakir 2006) (Rose 2021) (Lazarus 2010).
Even in the 16 page document, only six pages actually contained relevant data, as opposed to providing table of contents and other useless information.
